当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic insights into aryl nickel-catalyzed benzylic dehydrogenation of electron-deficient heteroarenes by using DFT calculations
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2021-08-06 , DOI: 10.1039/d1nj03119h
Feiyun Jia 1 , Bo Zhang 1
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2021-08-06 , DOI: 10.1039/d1nj03119h
Feiyun Jia 1 , Bo Zhang 1
Affiliation
|
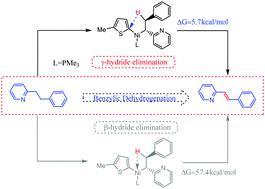
We recently investigated the mechanism of aryl nickel-catalyzed benzylic dehydrogenation of electron-deficient heteroarenes by using DFT calculations, which is an elegant protocol for accessing important precursor compounds such as 2-alkenyl heteroarenes. In this work, we proposed a novel γ-hydride elimination mechanism. Theoretical calculations supported our inference and ruled out the β-hydride elimination route proposed by the experimental group. Moreover, we found that the coordination of heterocyclic nitrogen to nickel metal is an important factor preventing the β-hydride elimination. Consistent with the experimental findings, the calculation conclusion confirmed that benzylic deprotonation is a reversible process. Additionally, we also found that a zinc salt plays an important role in affording the precursor of the transmetalation process, and the benzylic deprotonation is most likely to be a rate-determining step for this transformation. Finally, based on our calculations, we proposed an amended catalytic conversion mechanism.
中文翻译:

通过使用 DFT 计算对芳基镍催化的缺电子杂芳烃的苄脱氢的机理洞察
我们最近通过使用 DFT 计算研究了芳基镍催化的缺电子杂芳烃苄脱氢的机理,这是一种用于获取重要前体化合物(如 2-烯基杂芳烃)的优雅方案。在这项工作中,我们提出了一种新的γ-氢化物消除机制。理论计算支持了我们的推论,排除了实验组提出的β-氢化物消除路线。此外,我们发现杂环氮与镍金属的配位是阻止β-氢化物消除的重要因素。与实验结果一致,计算结论证实了苄基去质子化是一个可逆过程。此外,我们还发现锌盐在提供金属转移过程的前体方面起着重要作用,并且苄基去质子化最有可能是这种转化的速率决定步骤。最后,基于我们的计算,我们提出了一种修正的催化转化机制。
更新日期:2021-09-01
中文翻译:

通过使用 DFT 计算对芳基镍催化的缺电子杂芳烃的苄脱氢的机理洞察
我们最近通过使用 DFT 计算研究了芳基镍催化的缺电子杂芳烃苄脱氢的机理,这是一种用于获取重要前体化合物(如 2-烯基杂芳烃)的优雅方案。在这项工作中,我们提出了一种新的γ-氢化物消除机制。理论计算支持了我们的推论,排除了实验组提出的β-氢化物消除路线。此外,我们发现杂环氮与镍金属的配位是阻止β-氢化物消除的重要因素。与实验结果一致,计算结论证实了苄基去质子化是一个可逆过程。此外,我们还发现锌盐在提供金属转移过程的前体方面起着重要作用,并且苄基去质子化最有可能是这种转化的速率决定步骤。最后,基于我们的计算,我们提出了一种修正的催化转化机制。































 京公网安备 11010802027423号
京公网安备 11010802027423号