当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery and SAR Evolution of Pyrazole Azabicyclo[3.2.1]octane Sulfonamides as a Novel Class of Non-Covalent N-Acylethanolamine-Hydrolyzing Acid Amidase (NAAA) Inhibitors for Oral Administration
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-09-01 , DOI: 10.1021/acs.jmedchem.1c00575 Paolo Di Fruscia 1 , Anna Carbone 1, 2 , Giovanni Bottegoni 3 , Francesco Berti 1 , Francesca Giacomina 1 , Stefano Ponzano 1 , Chiara Pagliuca 1 , Annalisa Fiasella 1 , Daniela Pizzirani 1 , Jose Antonio Ortega 1 , Andrea Nuzzi 1 , Glauco Tarozzo 1 , Luisa Mengatto 1 , Roberta Giampà 1 , Ilaria Penna 1 , Debora Russo 1 , Elisa Romeo 4 , Maria Summa 5 , Rosalia Bertorelli 5 , Andrea Armirotti 5 , Sine Mandrup Bertozzi 5 , Angelo Reggiani 4 , Tiziano Bandiera 1 , Fabio Bertozzi 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-09-01 , DOI: 10.1021/acs.jmedchem.1c00575 Paolo Di Fruscia 1 , Anna Carbone 1, 2 , Giovanni Bottegoni 3 , Francesco Berti 1 , Francesca Giacomina 1 , Stefano Ponzano 1 , Chiara Pagliuca 1 , Annalisa Fiasella 1 , Daniela Pizzirani 1 , Jose Antonio Ortega 1 , Andrea Nuzzi 1 , Glauco Tarozzo 1 , Luisa Mengatto 1 , Roberta Giampà 1 , Ilaria Penna 1 , Debora Russo 1 , Elisa Romeo 4 , Maria Summa 5 , Rosalia Bertorelli 5 , Andrea Armirotti 5 , Sine Mandrup Bertozzi 5 , Angelo Reggiani 4 , Tiziano Bandiera 1 , Fabio Bertozzi 1
Affiliation
|
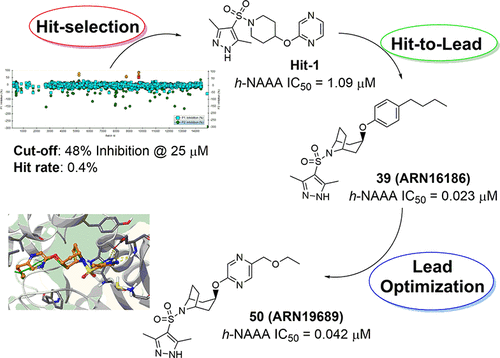
Inhibition of intracellular N-acylethanolamine-hydrolyzing acid amidase (NAAA) activity is a promising approach to manage the inflammatory response under disabling conditions. In fact, NAAA inhibition preserves endogenous palmitoylethanolamide (PEA) from degradation, thus increasing and prolonging its anti-inflammatory and analgesic efficacy at the inflamed site. In the present work, we report the identification of a potent, systemically available, novel class of NAAA inhibitors, featuring a pyrazole azabicyclo[3.2.1]octane structural core. After an initial screening campaign, a careful structure–activity relationship study led to the discovery of endo-ethoxymethyl-pyrazinyloxy-8-azabicyclo[3.2.1]octane-pyrazole sulfonamide 50 (ARN19689), which was found to inhibit human NAAA in the low nanomolar range (IC50 = 0.042 μM) with a non-covalent mechanism of action. In light of its favorable biochemical, in vitro and in vivo drug-like profile, sulfonamide 50 could be regarded as a promising pharmacological tool to be further investigated in the field of inflammatory conditions.
中文翻译:

作为一类新型口服非共价 N-酰基乙醇胺水解酰胺酶 (NAAA) 抑制剂的吡唑氮杂双环[3.2.1]辛烷磺酰胺的发现和 SAR 进化
抑制细胞内N-酰基乙醇胺水解酰胺酶 (NAAA) 活性是在致残条件下控制炎症反应的一种有前途的方法。事实上,NAAA 抑制作用可防止内源性棕榈酰乙醇酰胺 (PEA) 降解,从而增强并延长其在发炎部位的抗炎和镇痛功效。在目前的工作中,我们报告了一种有效的、系统可用的新型 NAAA 抑制剂的鉴定,其特征是吡唑氮杂双环[3.2.1]辛烷结构核心。经过初步筛选活动后,仔细的结构-活性关系研究发现了内-乙氧基甲基-吡嗪氧基-8-氮杂双环[3.2.1]辛烷-吡唑磺酰胺50 ( ARN19689 ),它被发现可以抑制人类 NAAA低纳摩尔范围(IC 50 = 0.042 μM),具有非共价作用机制。鉴于其良好的生化、体外和体内药物样特征,磺酰胺50可以被视为一种有前途的药理学工具,有待在炎症性疾病领域进行进一步研究。
更新日期:2021-09-23
中文翻译:

作为一类新型口服非共价 N-酰基乙醇胺水解酰胺酶 (NAAA) 抑制剂的吡唑氮杂双环[3.2.1]辛烷磺酰胺的发现和 SAR 进化
抑制细胞内N-酰基乙醇胺水解酰胺酶 (NAAA) 活性是在致残条件下控制炎症反应的一种有前途的方法。事实上,NAAA 抑制作用可防止内源性棕榈酰乙醇酰胺 (PEA) 降解,从而增强并延长其在发炎部位的抗炎和镇痛功效。在目前的工作中,我们报告了一种有效的、系统可用的新型 NAAA 抑制剂的鉴定,其特征是吡唑氮杂双环[3.2.1]辛烷结构核心。经过初步筛选活动后,仔细的结构-活性关系研究发现了内-乙氧基甲基-吡嗪氧基-8-氮杂双环[3.2.1]辛烷-吡唑磺酰胺50 ( ARN19689 ),它被发现可以抑制人类 NAAA低纳摩尔范围(IC 50 = 0.042 μM),具有非共价作用机制。鉴于其良好的生化、体外和体内药物样特征,磺酰胺50可以被视为一种有前途的药理学工具,有待在炎症性疾病领域进行进一步研究。






























 京公网安备 11010802027423号
京公网安备 11010802027423号