当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
AZD0284, a Potent, Selective, and Orally Bioavailable Inverse Agonist of Retinoic Acid Receptor-Related Orphan Receptor C2
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-08-31 , DOI: 10.1021/acs.jmedchem.1c01197 Frank Narjes , Antonio Llinas , Stefan von Berg , Johan Jirholt , Sarah Lever , Rikard Pehrson , Mia Collins , Anna Malmberg , Petter Svanberg , Yafeng Xue 1 , Roine I Olsson , Jesper Malmberg , Glyn Hughes , Nafizal Hossain , Hanna Grindebacke , Agnes Leffler , Nina Krutrök , Elisabeth Bäck , Marie Ramnegård , Matti Lepistö , Linda Thunberg 2 , Anna Aagaard 1 , Jane McPheat 1 , Eva L Hansson 1 , Rongfeng Chen 3 , Yao Xiong 3 , Thomas G Hansson
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-08-31 , DOI: 10.1021/acs.jmedchem.1c01197 Frank Narjes , Antonio Llinas , Stefan von Berg , Johan Jirholt , Sarah Lever , Rikard Pehrson , Mia Collins , Anna Malmberg , Petter Svanberg , Yafeng Xue 1 , Roine I Olsson , Jesper Malmberg , Glyn Hughes , Nafizal Hossain , Hanna Grindebacke , Agnes Leffler , Nina Krutrök , Elisabeth Bäck , Marie Ramnegård , Matti Lepistö , Linda Thunberg 2 , Anna Aagaard 1 , Jane McPheat 1 , Eva L Hansson 1 , Rongfeng Chen 3 , Yao Xiong 3 , Thomas G Hansson
Affiliation
|
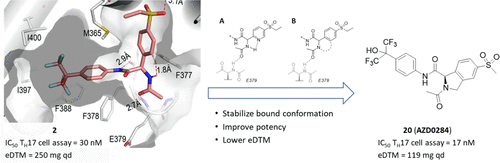
Inverse agonists of the nuclear receptor RORC2 have been widely pursued as a potential treatment for a variety of autoimmune diseases. We have discovered a novel series of isoindoline-based inverse agonists of the nuclear receptor RORC2, derived from our recently disclosed RORC2 inverse agonist 2. Extensive structure–activity relationship (SAR) studies resulted in AZD0284 (20), which combined potent inhibition of IL-17A secretion from primary human TH17 cells with excellent metabolic stability and good PK in preclinical species. In two preclinical in vivo studies, compound 20 reduced thymocyte numbers in mice and showed dose-dependent reduction of IL-17A containing γδ-T cells and of IL-17A and IL-22 RNA in the imiquimod induced inflammation model. Based on these data and a favorable safety profile, 20 was progressed to phase 1 clinical studies.
中文翻译:

AZD0284,一种有效的、选择性的、口服生物可利用的视黄酸受体相关孤儿受体 C2 反向激动剂
核受体 RORC2 的反向激动剂已被广泛视为多种自身免疫性疾病的潜在治疗方法。我们发现了一系列新型的基于异吲哚啉的核受体 RORC2 反向激动剂,源自我们最近公开的 RORC2 反向激动剂2 。广泛的构效关系 (SAR) 研究产生了 AZD0284 ( 20 ),它结合了对原代人 T H 17 细胞 IL-17A 分泌的有效抑制,在临床前物种中具有出色的代谢稳定性和良好的 PK。在两项临床前体内研究中,化合物20减少了小鼠胸腺细胞数量,并在咪喹莫特诱导的炎症模型中显示出含有 IL-17A 的 γδ-T 细胞以及 IL-17A 和 IL-22 RNA 的剂量依赖性减少。基于这些数据和良好的安全性, 20 项研究已进入 1 期临床研究。
更新日期:2021-09-23
中文翻译:

AZD0284,一种有效的、选择性的、口服生物可利用的视黄酸受体相关孤儿受体 C2 反向激动剂
核受体 RORC2 的反向激动剂已被广泛视为多种自身免疫性疾病的潜在治疗方法。我们发现了一系列新型的基于异吲哚啉的核受体 RORC2 反向激动剂,源自我们最近公开的 RORC2 反向激动剂2 。广泛的构效关系 (SAR) 研究产生了 AZD0284 ( 20 ),它结合了对原代人 T H 17 细胞 IL-17A 分泌的有效抑制,在临床前物种中具有出色的代谢稳定性和良好的 PK。在两项临床前体内研究中,化合物20减少了小鼠胸腺细胞数量,并在咪喹莫特诱导的炎症模型中显示出含有 IL-17A 的 γδ-T 细胞以及 IL-17A 和 IL-22 RNA 的剂量依赖性减少。基于这些数据和良好的安全性, 20 项研究已进入 1 期临床研究。






























 京公网安备 11010802027423号
京公网安备 11010802027423号