Tetrahedron Letters ( IF 1.5 ) Pub Date : 2021-09-01 , DOI: 10.1016/j.tetlet.2021.153386 Tohru Nakamura 1 , Tatsuya Ito 2
|
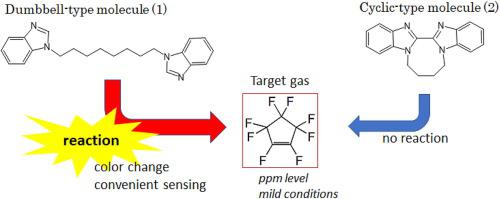
Sensing of octafluorocyclopentene (C5F8) with benzimidazole derivatives, i.e., dumbbell-type molecule (1) (1,8-Bis(1-benzimidazolyl)octane) and cyclic-type molecule (2) (1,1′-Tetramethylene-2,2′-bisbenzimidazole) were studied by UV-Visible, NMR spectroscopic and theoretical analyses from the viewpoint of applications to gas-sensors. Obtained results indicate a moderate reactivity of nucleophilic reactions toward C5F8 in the case of dumbbell-type molecule (1), on the other hand, no reactivity of cyclic-type molecule (2). Theoretical investigations suggest a decrease of electron density of imine group in cyclic-type molecule (2) as compared with that of dumbbell-type molecule (1). Dumbbell-type molecule (1) shows a ppm-level detection of C5F8 by UV-Visible and is not inhibited by inhibitor gases such as perfluorocarbons, ethanol, and vapor acid. The present reaction of molecule (1) will be conducive to a new type of sensing materials for unsaturated fluorocarbons with high sensitivity and selectivity in the field of environmental science and industrial etching process.
中文翻译:

哑铃型分子(1,8-双(1-苯并咪唑基)辛烷)和环状分子(1,1'-四亚甲基-2,2'-双苯并咪唑)对八氟环戊烯(C5F8)的传感特性和不同反应性
八氟环戊烯(C 5 F 8 ) 与苯并咪唑衍生物的感应,即哑铃型分子 (1) (1,8-双(1-苯并咪唑基)辛烷) 和环状分子 (2) (1,1'-四亚甲基) -2,2'-双苯并咪唑)从应用于气体传感器的角度通过紫外-可见光、核磁共振光谱和理论分析进行了研究。获得的结果表明对 C 5 F 8的亲核反应具有中等反应性另一方面,在哑铃型分子(1)的情况下,环状分子(2)没有反应性。理论研究表明,与哑铃型分子(1)相比,环状分子(2)中亚胺基团的电子密度降低。哑铃型分子 (1) 显示了通过紫外-可见光对 C 5 F 8的 ppm 级检测,并且不受抑制剂气体(例如全氟化碳、乙醇和蒸气酸)的抑制。目前分子(1)的反应将有利于环境科学和工业蚀刻工艺领域开发出一种具有高灵敏度和选择性的新型不饱和碳氟化合物传感材料。

































 京公网安备 11010802027423号
京公网安备 11010802027423号