当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Boosting Photoredox Catalysis Using a Two-Dimensional Electride as a Persistent Electron Donor
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-08-31 , DOI: 10.1021/acsami.1c12363 Seunga Heo 1, 2 , Yu Sung Chun 1 , Joonho Bang 3 , Ho Seong Hwang 4 , Sanju Hwang 1 , Sonam Kim 1 , Eun Jin Cho 4 , Sung Wng Kim 3, 5 , Youngmin You 1, 2
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-08-31 , DOI: 10.1021/acsami.1c12363 Seunga Heo 1, 2 , Yu Sung Chun 1 , Joonho Bang 3 , Ho Seong Hwang 4 , Sanju Hwang 1 , Sonam Kim 1 , Eun Jin Cho 4 , Sung Wng Kim 3, 5 , Youngmin You 1, 2
Affiliation
|
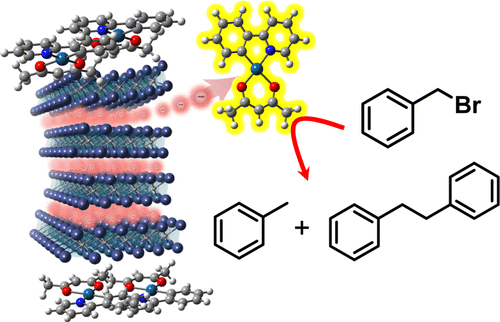
Electrides, which have excess anionic electrons, are solid-state sources of solvated electrons that can be used as powerful reducing agents for organic syntheses. However, the abrupt decomposition of electrides in organic solvents makes controlling the transfer inefficient, thereby limiting the utilization of their superior electron-donating ability. Here, we demonstrate the efficient reductive transformation strategy which combines the stable two-dimensional [Gd2C]2+·2e– electride electron donor and cyclometalated Pt(II) complex photocatalysts. Strongly localized anionic electrons at the interlayer space in the [Gd2C]2+·2e– electride are released via moderate alcoholysis in 2,2,2-trifluoroethanol, enabling persistent electron donation. The Pt(II) complexes are adsorbed onto the surface of the [Gd2C]2+·2e– electride and rapidly capture the released electrons at a rate of 107 s–1 upon photoexcitation. The one-electron-reduced Pt complex is electrochemically stable enough to deliver the electron to substrates in the bulk, which completes the photoredox cycle. The key benefit of this system is the suppression of undesirable charge recombination because back electron transfer is prohibited due to the irreversible disruption of the electride after the electron transfer. These desirable properties collectively serve as the photoredox catalysis principle for the reductive generation of the benzyl radical from benzyl halide, which is the key intermediate for dehalogenated or homocoupled products.
中文翻译:

使用二维电极作为持久电子供体促进光氧化还原催化
具有过量阴离子电子的电子是溶剂化电子的固态来源,可用作有机合成的强力还原剂。然而,电子化合物在有机溶剂中的突然分解使得控制转移效率低下,从而限制了其优越的给电子能力的利用。在这里,我们展示了结合稳定的二维 [Gd 2 C] 2+ ·2e -电子电子供体和环金属化 Pt(II) 配合物光催化剂的有效还原转化策略。[Gd 2 C] 2+ ·2e –层间空间的强定域阴离子电子通过在 2,2,2-三氟乙醇中适度醇解释放电子,从而实现持久的电子捐赠。Pt(II) 配合物吸附在 [Gd 2 C] 2+ ·2e –电子化物的表面,并以 10 7 s –1的速率快速捕获释放的电子在光激发时。单电子还原的 Pt 复合物在电化学上足够稳定,可以将电子大量传递到基板上,从而完成光氧化还原循环。该系统的主要优点是抑制了不需要的电荷复合,因为电子转移后电子化合物的不可逆破坏而禁止反向电子转移。这些理想的特性共同用作从苄基卤化物还原生成苄基自由基的光氧化还原催化原理,苄基卤化物是脱卤或均偶合产物的关键中间体。
更新日期:2021-09-15
中文翻译:

使用二维电极作为持久电子供体促进光氧化还原催化
具有过量阴离子电子的电子是溶剂化电子的固态来源,可用作有机合成的强力还原剂。然而,电子化合物在有机溶剂中的突然分解使得控制转移效率低下,从而限制了其优越的给电子能力的利用。在这里,我们展示了结合稳定的二维 [Gd 2 C] 2+ ·2e -电子电子供体和环金属化 Pt(II) 配合物光催化剂的有效还原转化策略。[Gd 2 C] 2+ ·2e –层间空间的强定域阴离子电子通过在 2,2,2-三氟乙醇中适度醇解释放电子,从而实现持久的电子捐赠。Pt(II) 配合物吸附在 [Gd 2 C] 2+ ·2e –电子化物的表面,并以 10 7 s –1的速率快速捕获释放的电子在光激发时。单电子还原的 Pt 复合物在电化学上足够稳定,可以将电子大量传递到基板上,从而完成光氧化还原循环。该系统的主要优点是抑制了不需要的电荷复合,因为电子转移后电子化合物的不可逆破坏而禁止反向电子转移。这些理想的特性共同用作从苄基卤化物还原生成苄基自由基的光氧化还原催化原理,苄基卤化物是脱卤或均偶合产物的关键中间体。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号