Separation and Purification Technology ( IF 8.1 ) Pub Date : 2021-08-25 , DOI: 10.1016/j.seppur.2021.119559 Abolfazl Ahmadi 1 , Mahmoud Zarei 1 , Aydin Hassani 2 , Masoud Ebratkhahan 1 , Ali Olad 3
|
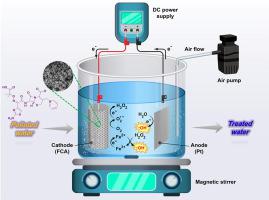
In the present study, the electro-Fenton (EF) process has been used to remove ceftazidime (CAZ) from an aqueous solution. In the first part of this study, the iron (II) doped carbonaceous aerogel (denote as FCA) as a three-dimensional cathode was synthesized as a cathode electrode. For this purpose, resorcinol-formaldehyde polymers containing iron (II) salt were prepared by sol-gel method and after drying by ambient drying method, the resulting polymer was finally carbonized in a furnace with inert nitrogen medium at 800°C and the resulting composite properties examined by Field Emission Scanning Electron Microscopy, Energy Dispersive X-Ray, Brunaur-Emmett-Teller, X-Ray Diffraction, Fourier Transform Infrared Spectroscopy, and cyclic voltammetry analyses. In the last part of this study, the prepared carbonaceous aerogel was employed as a cathode electrode in the EF system to remove CAZ from an aqueous medium. The influence of key factors such as applied current (mA), initial pH of the solution, initial CAZ concentration (mg/L), and process time (min) on the CAZ removal efficiency (RE (%)) under the EF process was assessed. The mineralization current efficiency (MCE) was found as 60.66% in 200 min. Response surface methodology (RSM) was used to optimize the process. The results showed that the optimal values for the variables of applied current intensity, pH, initial CAZ concentration, and process time were 400 mA, 4, 40 mg/L, and 110 min, respectively. The intermediates of CAZ removal were identified by gas chromatography-mass spectrometry (GC-MS) analysis. Moreover, the possible removal mechanism of CAZ was proposed. The reusability and stability of the FCA cathode was assessed and revealed an approximately 4% decline in CAZ removal efficiency after eight consecutive cycles. The amount of organic carbon degraded by this process was investigated by chemical oxygen demand (COD) test and it was 99% after 200 min of reaction time.
中文翻译:

铁(II)掺杂碳质气凝胶作为三维阴极的简便合成及其在水溶液中电芬顿降解头孢他啶的优异性能
在本研究中,电芬顿 (EF) 工艺已用于从水溶液中去除头孢他啶 (CAZ)。在本研究的第一部分,铁(II)掺杂的碳质气凝胶(表示为 FCA)作为三维阴极被合成为阴极。为此,采用溶胶-凝胶法制备含铁(II)盐的间苯二酚-甲醛聚合物,并通过常温干燥法干燥后,最终将所得聚合物在惰性氮气介质中于 800°C 的炉中进行碳化,并得到所得复合材料通过场发射扫描电子显微镜、能量色散 X 射线、Brunaur-Emmett-Teller、X 射线衍射、傅里叶变换红外光谱和循环伏安法分析检查的特性。在本研究的最后一部分,制备的碳质气凝胶用作 EF 系统中的阴极电极,以从水性介质中去除 CAZ。EF工艺下施加电流(mA)、溶液初始pH值、初始CAZ浓度(mg/L)和处理时间(min)等关键因素对CAZ去除效率(RE(%))的影响为评估。在 200 分钟内发现矿化电流效率 (MCE) 为 60.66%。响应面方法 (RSM) 用于优化该过程。结果表明,施加电流强度、pH、初始 CAZ 浓度和处理时间等变量的最佳值分别为 400 mA、4、40 mg/L 和 110 min。通过气相色谱-质谱 (GC-MS) 分析鉴定去除 CAZ 的中间体。此外,提出了CAZ可能的去除机制。对 FCA 阴极的可重复使用性和稳定性进行了评估,结果显示连续八次循环后 CAZ 去除效率下降约 4%。通过化学需氧量(COD)测试研究了该过程降解的有机碳量,反应时间为 200 分钟后为 99%。

































 京公网安备 11010802027423号
京公网安备 11010802027423号