当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficiently Enantioselective Hydrogenation Photosynthesis of (R)-1-[3,5-Bis(trifluoromethyl)phenyl] ethanol over a CLEs-TiO2 Bioinorganic Hybrid Materials
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-08-25 , DOI: 10.1021/acsami.1c11050 Youcheng Yin 1, 2, 3 , Ru Wang 1, 2, 3 , Jing Zhang 1 , Zhiyuan Luo 1 , Qinjie Xiao 1 , Tian Xie 2, 3 , Xiaolin Pei 1 , Peng Gao 1 , Anming Wang 1, 3
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-08-25 , DOI: 10.1021/acsami.1c11050 Youcheng Yin 1, 2, 3 , Ru Wang 1, 2, 3 , Jing Zhang 1 , Zhiyuan Luo 1 , Qinjie Xiao 1 , Tian Xie 2, 3 , Xiaolin Pei 1 , Peng Gao 1 , Anming Wang 1, 3
Affiliation
|
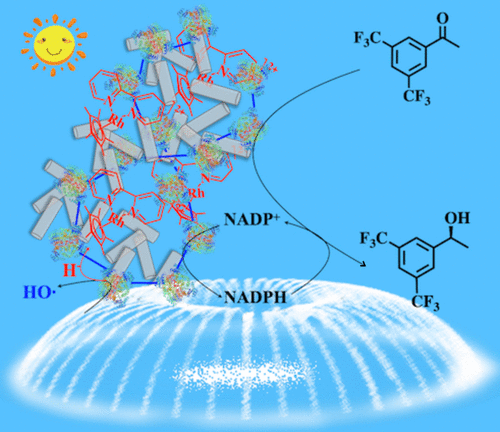
Engineering of biological pathways with man-made materials provides inspiring blueprints for sustainable drug production. (R)-1-[3,5-Bis(trifluoromethyl)phenyl]ethanol [(R)-3,5-BTPE], as an important artificial chiral intermediate for complicated pharmaceutical drugs and biologically active molecules, is often synthesized through a hydrogenation reaction of 3,5-bis(trifluoromethyl)acetophenone (3,5-BTAP), in which enantioselectivity and sufficient active hydrogen are the key to restricting the reaction. In this work, a biohybrid photocatalytic hydrogenation system based on an artificial cross-linked enzymes (CLEs)-TiO2-Cp*Rh(bpy) photoenzyme is developed through a bottom-up engineering strategy. Here, TiO2 nanotubes in the presence of Cp*Rh(bpy) are used to transform NADP+ to NADPH during the formation of chiral alcohol intermediates from the catalytic reduction of a ketone substrate by alcohol dehydrogenase CLEs. Hydrogen and electrons, provided by water and photocatalytic systems, respectively, are transferred to reduce NADP+ to NADPH via [Cp*Rh(bpy)(H2O)]2+. With the resulting NADPH, [(R)-3,5-BTPE] is synthesized using our efficient CLEs obtained from the cell lysate by nonstandard amino acid modification. Through this biohybrid photocatalytic system, the photoenzyme-catalyzed combined reductive synthesis of [(R)-3,5-BTPE] has a yield of 41.2% after reaction for 24 h and a very high enantiomeric excess value (>99.99%). In the case of reuse, this biohybrid system retained nearly 95% of its initial catalytic activity for synthesizing the above chiral alcohol. The excellent reusability of the CLEs and TiO2 nanotubes hybrid catalytic materials highlights the environmental friendliness of (R)-3,5-BTPE production.
中文翻译:

(R)-1-[3,5-双(三氟甲基)苯基]乙醇在 CLEs-TiO2 生物无机杂化材料上的高效对映选择性加氢光合作用
使用人造材料进行生物通路工程为可持续药物生产提供了鼓舞人心的蓝图。( R )-1-[3,5-双(三氟甲基)苯基]乙醇 [( R )-3,5-BTPE] 作为复杂药物和生物活性分子的重要人工手性中间体,通常通过合成3,5-双(三氟甲基)苯乙酮(3,5-BTAP)的加氢反应,其中对映选择性和足够的活性氢是限制反应的关键。在这项工作中,通过自下而上的工程策略开发了一种基于人工交联酶(CLEs)-TiO 2 -Cp*Rh(bpy) 光酶的生物混合光催化加氢系统。这里,TiO 2在 Cp*Rh(bpy) 存在下的纳米管用于在醇脱氢酶 CLE 催化还原酮底物形成手性醇中间体期间将 NADP +转化为 NADPH。分别由水和光催化系统提供的氢和电子通过[Cp*Rh(bpy)(H 2 O)] 2+转移以将NADP +还原为NADPH 。使用所得的 NADPH,使用我们通过非标准氨基酸修饰从细胞裂解物中获得的高效 CLE 合成 [( R )-3,5-BTPE]。通过这种生物混合光催化体系,光酶催化的联合还原合成[( R)-3,5-BTPE] 反应 24 小时后的产率为 41.2%,对映体过量值非常高 (>99.99%)。在重复使用的情况下,这种生物混合系统保留了近 95% 的初始催化活性,用于合成上述手性醇。CLEs和TiO 2纳米管混合催化材料优异的可重复使用性突出了( R )-3,5-BTPE生产的环境友好性。
更新日期:2021-09-08
中文翻译:

(R)-1-[3,5-双(三氟甲基)苯基]乙醇在 CLEs-TiO2 生物无机杂化材料上的高效对映选择性加氢光合作用
使用人造材料进行生物通路工程为可持续药物生产提供了鼓舞人心的蓝图。( R )-1-[3,5-双(三氟甲基)苯基]乙醇 [( R )-3,5-BTPE] 作为复杂药物和生物活性分子的重要人工手性中间体,通常通过合成3,5-双(三氟甲基)苯乙酮(3,5-BTAP)的加氢反应,其中对映选择性和足够的活性氢是限制反应的关键。在这项工作中,通过自下而上的工程策略开发了一种基于人工交联酶(CLEs)-TiO 2 -Cp*Rh(bpy) 光酶的生物混合光催化加氢系统。这里,TiO 2在 Cp*Rh(bpy) 存在下的纳米管用于在醇脱氢酶 CLE 催化还原酮底物形成手性醇中间体期间将 NADP +转化为 NADPH。分别由水和光催化系统提供的氢和电子通过[Cp*Rh(bpy)(H 2 O)] 2+转移以将NADP +还原为NADPH 。使用所得的 NADPH,使用我们通过非标准氨基酸修饰从细胞裂解物中获得的高效 CLE 合成 [( R )-3,5-BTPE]。通过这种生物混合光催化体系,光酶催化的联合还原合成[( R)-3,5-BTPE] 反应 24 小时后的产率为 41.2%,对映体过量值非常高 (>99.99%)。在重复使用的情况下,这种生物混合系统保留了近 95% 的初始催化活性,用于合成上述手性醇。CLEs和TiO 2纳米管混合催化材料优异的可重复使用性突出了( R )-3,5-BTPE生产的环境友好性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号