当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photoredox/nickel dual-catalyzed regioselective alkylation of propargylic carbonates for trisubstituted allenes
Chemical Communications ( IF 4.3 ) Pub Date : 2021-08-09 , DOI: 10.1039/d1cc03303d Zhao-Zhao Zhou 1, 2 , Xian-Rong Song 3 , Sha Du 3 , Ke-Jian Xia 1 , Wan-Fa Tian 3 , Qiang Xiao 3 , Yong-Min Liang 2
Chemical Communications ( IF 4.3 ) Pub Date : 2021-08-09 , DOI: 10.1039/d1cc03303d Zhao-Zhao Zhou 1, 2 , Xian-Rong Song 3 , Sha Du 3 , Ke-Jian Xia 1 , Wan-Fa Tian 3 , Qiang Xiao 3 , Yong-Min Liang 2
Affiliation
|
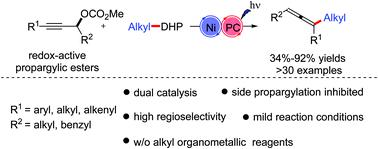
Herein, a highly regioselective alkylation of propargylic carbonates for trisubstituted allenes with alkyl 1,4-dihydropyridine derivatives (1,4-DHPs) is developed via a photoredox/nickel dual-catalyzed process, which represents the first direct approach to access alkylated allene products without alkyl organometallic reagents. This method features a broad substrate scope and mild conditions. A hypothetical mechanism with an alkyl radical and an allenyl Ni(III) species is proposed. Benzylation products were also obtained to be the complement building blocks for the potential synthesis of pharmaceuticals.
中文翻译:

用于三取代丙二烯的炔丙基碳酸酯的光氧化还原/镍双催化区域选择性烷基化
在此,通过光氧化还原/镍双催化工艺开发了具有烷基 1,4-二氢吡啶衍生物(1,4-DHP)的三取代丙二烯的炔丙碳酸酯的高度区域选择性烷基化,这是获得烷基化丙二烯产物的第一种直接方法不含烷基有机金属试剂。该方法具有广泛的底物范围和温和的条件。提出了一种具有烷基和烯丙基 Ni( III ) 物质的假设机制。还获得了苄基化产物,作为药物潜在合成的补充构件。
更新日期:2021-08-24
中文翻译:

用于三取代丙二烯的炔丙基碳酸酯的光氧化还原/镍双催化区域选择性烷基化
在此,通过光氧化还原/镍双催化工艺开发了具有烷基 1,4-二氢吡啶衍生物(1,4-DHP)的三取代丙二烯的炔丙碳酸酯的高度区域选择性烷基化,这是获得烷基化丙二烯产物的第一种直接方法不含烷基有机金属试剂。该方法具有广泛的底物范围和温和的条件。提出了一种具有烷基和烯丙基 Ni( III ) 物质的假设机制。还获得了苄基化产物,作为药物潜在合成的补充构件。































 京公网安备 11010802027423号
京公网安备 11010802027423号