当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Seven-Step Total Synthesis of Sporothriolide
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-08-23 , DOI: 10.1021/acs.joc.1c01663 Miku Kimura 1 , Tomoyo Mohri 1 , Masaru Enomoto 1 , Yasuhiro Meguro 1 , Yusuke Ogura 1 , Shigefumi Kuwahara 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-08-23 , DOI: 10.1021/acs.joc.1c01663 Miku Kimura 1 , Tomoyo Mohri 1 , Masaru Enomoto 1 , Yasuhiro Meguro 1 , Yusuke Ogura 1 , Shigefumi Kuwahara 1
Affiliation
|
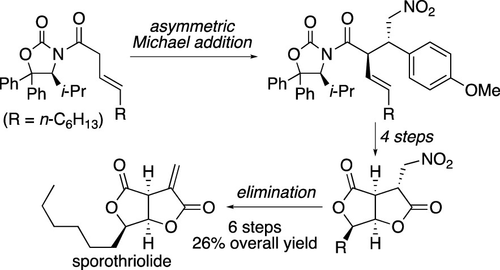
An enantioselective total synthesis of sporothriolide, a bioactive furofurandione-type fungal metabolite, has been achieved in a 21% overall yield from a commercially available β,γ-unsaturated carboxylic acid via seven steps. The key steps of this synthesis include a highly diastereoselective Michael addition of a chiral oxazolidinone derivative to a nitro olefin, the exploitation of an aromatic ring as a masked carboxylic acid functionality, and the base-promoted elimination of nitrous acid to install the α-methylene lactone unit of sporothriolide in the final step.
中文翻译:

孢子丝菌素的七步全合成
孢子丝菌内酯是一种具有生物活性的呋喃二酮类真菌代谢物,通过七个步骤从市售的 β,γ-不饱和羧酸中以 21% 的总产率实现了对映选择性全合成。该合成的关键步骤包括手性恶唑烷酮衍生物与硝基烯烃的高度非对映选择性迈克尔加成,利用芳环作为掩蔽的羧酸官能团,以及亚硝酸的碱促进消除以安装 α-亚甲基最后一步中的孢子丝菌内酯单元。
更新日期:2021-09-03
中文翻译:

孢子丝菌素的七步全合成
孢子丝菌内酯是一种具有生物活性的呋喃二酮类真菌代谢物,通过七个步骤从市售的 β,γ-不饱和羧酸中以 21% 的总产率实现了对映选择性全合成。该合成的关键步骤包括手性恶唑烷酮衍生物与硝基烯烃的高度非对映选择性迈克尔加成,利用芳环作为掩蔽的羧酸官能团,以及亚硝酸的碱促进消除以安装 α-亚甲基最后一步中的孢子丝菌内酯单元。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号