当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis, and biological evaluation of benzo[b]thiophene 1,1-dioxide derivatives as potent STAT3 inhibitors
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2021-08-20 , DOI: 10.1111/cbdd.13939 Wen‐Zhen Li 1 , Hui‐Zhi Xi 1 , Yi‐Jie Wang 1 , Hong‐Bo Ma 2 , Zhi‐Qiang Cheng 3 , Yu Yang 1 , Meng‐Ling Wu 1 , Ting‐Mei Liu 1 , Wen Yang 1 , Qin Wang 1 , Meng‐Ya Liao 4 , Yong Xia 1 , Yi‐Wen Zhang 1
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2021-08-20 , DOI: 10.1111/cbdd.13939 Wen‐Zhen Li 1 , Hui‐Zhi Xi 1 , Yi‐Jie Wang 1 , Hong‐Bo Ma 2 , Zhi‐Qiang Cheng 3 , Yu Yang 1 , Meng‐Ling Wu 1 , Ting‐Mei Liu 1 , Wen Yang 1 , Qin Wang 1 , Meng‐Ya Liao 4 , Yong Xia 1 , Yi‐Wen Zhang 1
Affiliation
|
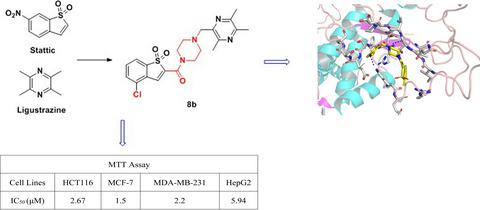
As a member of the signal transducer and activator of transcription (STAT) family, STAT3 plays a critical role in several biological pathways such as cell proliferation, migration, survival, and differentiation. Due to abnormal continuous activation in tumors, inhibition of STAT3 has emerged as an attractive approach for the treatment of various cancer cells. Herein, we report a series of novel STAT3 inhibitors based on benzo[b]thiophene 1,1-dioxide scaffold and evaluated their anticancer potency. Among them, compound 8b exhibited the best activity against cancer cells. Compound 8b induced apoptosis and blocked the cell cycle. Meanwhile, 8b reduced intracellular ROS content and caused the loss of mitochondrial membrane potential. Further research revealed that 8b significantly blocked STAT3 phosphorylation and STAT3-dependent dual-luciferase reporter gene experiments showed that compound 8b has a marked inhibition of STAT3-mediated Firefly luciferase activity. Molecular modeling studies revealed compound 8b occupied the pocket well with the SH2 domain in a favorable conformation.
中文翻译:

作为有效的 STAT3 抑制剂的苯并 [b] 噻吩 1,1-二氧化物衍生物的设计、合成和生物学评价
作为信号转导和转录激活因子 (STAT) 家族的成员,STAT3 在细胞增殖、迁移、存活和分化等多种生物学途径中发挥着关键作用。由于肿瘤中异常的持续激活,抑制 STAT3 已成为治疗各种癌细胞的有吸引力的方法。在此,我们报告了一系列基于苯并 [b] 噻吩 1,1-二氧化物支架的新型 STAT3 抑制剂,并评估了它们的抗癌效力。其中,化合物8b对癌细胞的活性最好。化合物8b诱导细胞凋亡并阻断细胞周期。同时,8b降低细胞内 ROS 含量并导致线粒体膜电位的丧失。进一步研究表明,8b显着阻断 STAT3 磷酸化,STAT3 依赖性双荧光素酶报告基因实验表明,化合物8b对 STAT3 介导的萤火虫荧光素酶活性有显着抑制作用。分子建模研究表明,化合物8b以有利的构象占据了具有 SH2 结构域的口袋。
更新日期:2021-10-29
中文翻译:

作为有效的 STAT3 抑制剂的苯并 [b] 噻吩 1,1-二氧化物衍生物的设计、合成和生物学评价
作为信号转导和转录激活因子 (STAT) 家族的成员,STAT3 在细胞增殖、迁移、存活和分化等多种生物学途径中发挥着关键作用。由于肿瘤中异常的持续激活,抑制 STAT3 已成为治疗各种癌细胞的有吸引力的方法。在此,我们报告了一系列基于苯并 [b] 噻吩 1,1-二氧化物支架的新型 STAT3 抑制剂,并评估了它们的抗癌效力。其中,化合物8b对癌细胞的活性最好。化合物8b诱导细胞凋亡并阻断细胞周期。同时,8b降低细胞内 ROS 含量并导致线粒体膜电位的丧失。进一步研究表明,8b显着阻断 STAT3 磷酸化,STAT3 依赖性双荧光素酶报告基因实验表明,化合物8b对 STAT3 介导的萤火虫荧光素酶活性有显着抑制作用。分子建模研究表明,化合物8b以有利的构象占据了具有 SH2 结构域的口袋。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号