当前位置:
X-MOL 学术
›
Pharmacol. Res. Perspect.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phase 1 study in healthy participants of the safety, pharmacokinetics, and pharmacodynamics of enpatoran (M5049), a dual antagonist of toll-like receptors 7 and 8
Pharmacology Research & Perspectives ( IF 2.9 ) Pub Date : 2021-08-19 , DOI: 10.1002/prp2.842
Andreas Port 1 , Jamie V Shaw 2 , Lena Klopp-Schulze 1 , Afrim Bytyqi 1 , Claudia Vetter 1 , Elizabeth Hussey 3 , Nadra Mammasse 4 , Victor Ona 2 , Angelika Bachmann 1 , Denis Strugala 5 , Christian Reh 5 , Kosalaram Goteti 2
Pharmacology Research & Perspectives ( IF 2.9 ) Pub Date : 2021-08-19 , DOI: 10.1002/prp2.842
Andreas Port 1 , Jamie V Shaw 2 , Lena Klopp-Schulze 1 , Afrim Bytyqi 1 , Claudia Vetter 1 , Elizabeth Hussey 3 , Nadra Mammasse 4 , Victor Ona 2 , Angelika Bachmann 1 , Denis Strugala 5 , Christian Reh 5 , Kosalaram Goteti 2
Affiliation
|
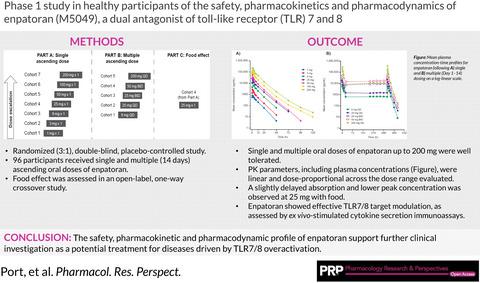
This study evaluated the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of single and multiple oral doses of enpatoran (formerly named M5049), a new toll-like receptor (TLR) 7 and 8 dual antagonist, and the effect of food on a single dose in healthy participants. In this single phase 1, randomized (3:1), double-blind, placebo-controlled study, 96 participants received single and multiple ascending oral doses of enpatoran. Participants in single-dose cohorts received one dose of enpatoran (1, 3, 9, 25, 50, 100, or 200 mg) or placebo using a sentinel dosing strategy. Multiple-dose cohorts received enpatoran (9, 25, or 200 mg once daily, or 25 or 50 mg twice daily) or placebo for 14 days. Safety, tolerability, PK, and PD (ex vivo-stimulated cytokine secretion) were assessed in both parts. The effect of food was assessed in an open-label, one-way crossover study in the 25 mg single-dose cohort. Single- and multiple-oral doses of enpatoran up to 200 mg were well tolerated and no significant dose-limiting adverse events or safety signals were observed under fasting or fed conditions. PK parameters were linear and dose-proportional across the dose range evaluated, with a slightly delayed absorption and lower peak concentration observed at 25 mg with food. Exposure-dependent inhibition of ex vivo-stimulated interleukin-6 secretion was observed, with maximum inhibition at 200 mg. Enpatoran was well tolerated at doses up to 200 mg. Further investigation of enpatoran is warranted as a potential treatment for diseases driven by TLR7/8 overactivation, such as systemic lupus erythematosus and COVID-19 pneumonia.
中文翻译:

在健康受试者中开展的关于 Toll 样受体 7 和 8 双重拮抗剂 enpatoran (M5049) 安全性、药代动力学和药效学的 1 期研究
本研究评估了新型Toll样受体(TLR)7和8双重拮抗剂enpatoran(原名M5049)单次和多次口服剂量的安全性、耐受性、药代动力学(PK)和药效学(PD)以及效果健康参与者单剂量食物的摄入量。在这项单期 1 期、随机 (3:1)、双盲、安慰剂对照研究中,96 名参与者接受了单次和多次递增口服剂量的 enpatoran。单剂量队列的参与者使用哨兵给药策略接受一剂 enpatoran(1、3、9、25、50、100 或 200 mg)或安慰剂。多剂量组接受 enpatoran(9、25 或 200 mg 每日一次,或 25 或 50 mg 每日两次)或安慰剂治疗 14 天。安全性、耐受性、PK 和 PD(体外刺激的细胞因子分泌)在两个部分均进行了评估。在一项开放标签、单向交叉研究中,在 25 毫克单剂量队列中评估了食物的影响。单次和多次口服剂量高达 200 mg 的 enpatoran 耐受性良好,在禁食或进食条件下未观察到明显的剂量限制性不良事件或安全信号。在评估的剂量范围内,PK 参数呈线性且与剂量成比例,在 25 mg 与食物一起服用时观察到吸收略有延迟和较低的峰值浓度。观察到对离体刺激的白细胞介素 6 分泌的暴露依赖性抑制,在 200 mg 时达到最大抑制。 Enpatoran 在剂量高达 200 mg 时具有良好的耐受性。需要对 enpatoran 进行进一步研究,将其作为 TLR7/8 过度激活引起的疾病(例如系统性红斑狼疮和 COVID-19 肺炎)的潜在治疗方法。
更新日期:2021-08-20
中文翻译:

在健康受试者中开展的关于 Toll 样受体 7 和 8 双重拮抗剂 enpatoran (M5049) 安全性、药代动力学和药效学的 1 期研究
本研究评估了新型Toll样受体(TLR)7和8双重拮抗剂enpatoran(原名M5049)单次和多次口服剂量的安全性、耐受性、药代动力学(PK)和药效学(PD)以及效果健康参与者单剂量食物的摄入量。在这项单期 1 期、随机 (3:1)、双盲、安慰剂对照研究中,96 名参与者接受了单次和多次递增口服剂量的 enpatoran。单剂量队列的参与者使用哨兵给药策略接受一剂 enpatoran(1、3、9、25、50、100 或 200 mg)或安慰剂。多剂量组接受 enpatoran(9、25 或 200 mg 每日一次,或 25 或 50 mg 每日两次)或安慰剂治疗 14 天。安全性、耐受性、PK 和 PD(体外刺激的细胞因子分泌)在两个部分均进行了评估。在一项开放标签、单向交叉研究中,在 25 毫克单剂量队列中评估了食物的影响。单次和多次口服剂量高达 200 mg 的 enpatoran 耐受性良好,在禁食或进食条件下未观察到明显的剂量限制性不良事件或安全信号。在评估的剂量范围内,PK 参数呈线性且与剂量成比例,在 25 mg 与食物一起服用时观察到吸收略有延迟和较低的峰值浓度。观察到对离体刺激的白细胞介素 6 分泌的暴露依赖性抑制,在 200 mg 时达到最大抑制。 Enpatoran 在剂量高达 200 mg 时具有良好的耐受性。需要对 enpatoran 进行进一步研究,将其作为 TLR7/8 过度激活引起的疾病(例如系统性红斑狼疮和 COVID-19 肺炎)的潜在治疗方法。

































 京公网安备 11010802027423号
京公网安备 11010802027423号