Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2021-08-20 , DOI: 10.1016/j.bmcl.2021.128335 Jason R Zbieg 1 , Jun Liang 1 , Jun Li 1 , Robert A Blake 1 , Jae Chang 1 , Lori Friedman 1 , Simon Goodacre 2 , Steven J Hartman 1 , Ellen Rei Ingalla 1 , James R Kiefer 1 , Tracy Kleinheinz 1 , Sharada Labadie 1 , Tommy Lai 3 , Jiangpeng Liao 3 , Nev McLean 2 , Ciara Metcalfe 1 , Vidhi Mody 1 , Michelle Nannini 1 , Daniel F Ortwine 1 , Yingqing Ran 1 , Nick Ray 2 , Fabien Roussel 2 , Amy Sambrone 1 , Deepak Sampath 1 , Maia Vinogradova 1 , John Wai 3 , Tao Wang 3 , Kuen Yeap 2 , Birong Zhang 1 , Xiaoping Zheng 3 , Yu Zhong 1 , Xiaojing Wang 1
|
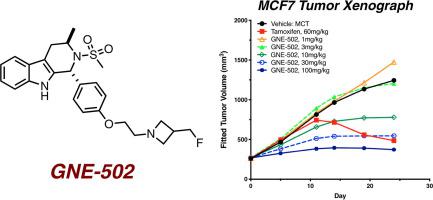
Fulvestrant is an FDA-approved drug with a dual mechanism of action (MOA), acting as a full antagonist and degrader of the estrogen receptor protein. A significant limitation of fulvestrant is the dosing regimen required for efficacy. Due to its high lipophilicity and poor pharmacokinetic profile, fulvestrant needs to be administered through intramuscular injections which leads to injection site soreness. This route of administration also limits the dose and target occupancy in patients. We envisioned a best-in-class molecule that would function with the same dual MOA as fulvestrant, but with improved physicochemical properties and would be orally bioavailable. Herein we report our progress toward that goal, resulting in a new lead GNE-502 which addressed some of the liabilities of our previously reported lead molecule GNE-149.
中文翻译:

发现 GNE-502 作为雌激素受体阳性乳腺癌的口服生物可利用和强效降解剂
Fulvestrant 是 FDA 批准的具有双重作用机制 (MOA) 的药物,可作为雌激素受体蛋白的完全拮抗剂和降解剂。氟维司群的一个显着限制是疗效所需的给药方案。由于其高亲脂性和较差的药代动力学特征,氟维司群需要通过肌肉注射给药,这会导致注射部位疼痛。这种给药途径还限制了患者的剂量和目标占有率。我们设想了一种一流的分子,该分子具有与氟维司群相同的双重 MOA 功能,但具有改进的物理化学特性,并且具有口服生物利用度。在此,我们报告了我们朝着这一目标取得的进展,产生了一种新的先导 GNE-502,它解决了我们之前报道的先导分子 GNE-149 的一些问题。































 京公网安备 11010802027423号
京公网安备 11010802027423号