当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solubility Behavior of l-Homophenylalanine Ethyl Ester Hydrochloride in 12 Individual Solvents from 283.15 to 323.15 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-08-20 , DOI: 10.1021/acs.jced.1c00485 Ying Guo , Hui He , Haishuang Huang , Jingxuan Qiu , Jiaming Han , Shen Hu , Haoyou Liu , Yue Zhao , Peng Wang
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-08-20 , DOI: 10.1021/acs.jced.1c00485 Ying Guo , Hui He , Haishuang Huang , Jingxuan Qiu , Jiaming Han , Shen Hu , Haoyou Liu , Yue Zhao , Peng Wang
|
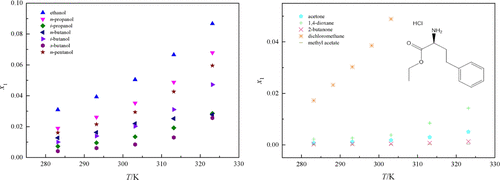
The mole fraction solubility data of l-homophenylalanine ethyl ester hydrochloride in 12 neat solvents (ethanol, n-propanol, i-propanol, n-butanol, i-butanol, s-butanol, n-pentanol, acetone, 1,4-dioxane, 2-butanone, dichloromethane, and methyl acetate) were determined by the static gravimetric method within the temperature range from 283.15 to 323.15 K under the ambient pressure of 98.8 kPa. Analysis of the neat solvents data showed that the solubility behavior was influenced by the molecular structure, molecular steric hindrance, polarity, hydrogen bond, cohesive energy density, and so on. Two thermodynamic models, i.e., the modified Apelblat model and the Yaws model were used to correlate the solubility data. To evaluate the fitting results, the average relative deviations, root mean square deviations, Akaike information criterion, and Akaike weights were calculated.
中文翻译:

L-高苯丙氨酸乙酯盐酸盐在 283.15 至 323.15 K 的 12 种单独溶剂中的溶解度行为
的摩尔分数溶解度数据升在12种整齐溶剂(乙醇, -高苯丙氨酸乙酯盐酸盐Ñ丙醇,我丙醇,Ñ丁醇,我丁醇,小号丁醇,Ñ-戊醇、丙酮、1,4-二恶烷、2-丁酮、二氯甲烷和乙酸甲酯)在283.15-323.15 K的温度范围内,在98.8 kPa的环境压力下通过静态重量法测定。对纯溶剂数据的分析表明,溶解行为受分子结构、分子位阻、极性、氢键、内聚能密度等的影响。两个热力学模型,即修正的Apelblat 模型和Yaws 模型用于关联溶解度数据。为了评估拟合结果,计算了平均相对偏差、均方根偏差、Akaike 信息准则和 Akaike 权重。
更新日期:2021-09-09
中文翻译:

L-高苯丙氨酸乙酯盐酸盐在 283.15 至 323.15 K 的 12 种单独溶剂中的溶解度行为
的摩尔分数溶解度数据升在12种整齐溶剂(乙醇, -高苯丙氨酸乙酯盐酸盐Ñ丙醇,我丙醇,Ñ丁醇,我丁醇,小号丁醇,Ñ-戊醇、丙酮、1,4-二恶烷、2-丁酮、二氯甲烷和乙酸甲酯)在283.15-323.15 K的温度范围内,在98.8 kPa的环境压力下通过静态重量法测定。对纯溶剂数据的分析表明,溶解行为受分子结构、分子位阻、极性、氢键、内聚能密度等的影响。两个热力学模型,即修正的Apelblat 模型和Yaws 模型用于关联溶解度数据。为了评估拟合结果,计算了平均相对偏差、均方根偏差、Akaike 信息准则和 Akaike 权重。












































 京公网安备 11010802027423号
京公网安备 11010802027423号