当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxygen Evolution Reaction on the Fe3O4(001) Surface: Theoretical Insights into the Role of Terminal and Bridging Oxygen Atoms
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2021-08-18 , DOI: 10.1021/acs.jpcc.1c05804 Giulia Righi 1 , Stefano Fabris 1 , Simone Piccinin 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2021-08-18 , DOI: 10.1021/acs.jpcc.1c05804 Giulia Righi 1 , Stefano Fabris 1 , Simone Piccinin 1
Affiliation
|
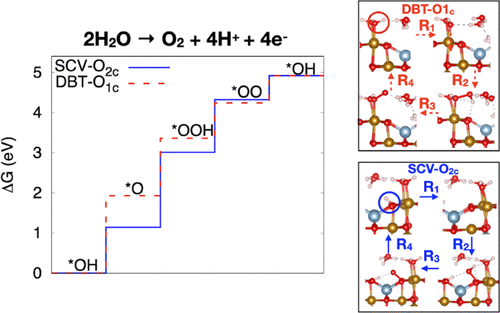
Model catalysts, where the structure of single-crystal materials with well-defined surface terminations can be determined at the atomic level, are useful systems to perform fundamental studies on electrocatalysis. The magnetite Fe3O4(001) surface, in particular, has been the focus of several studies aimed at characterizing its structure in vacuum in the presence of a water layer and interfaced with liquid water-based electrolytes. Recently, this system has also been investigated as a catalyst for the oxygen evolution reaction (OER), with the goal of correlating structural properties of the interface with catalytic performance and mechanism. In this work, we use first-principles simulations based on density functional theory to establish the structural, thermodynamic, and electronic properties of the Fe3O4(001) surface in contact with water. We compute the phase diagram of the magnetite/water system and address some open issues on the structural transitions observed experimentally among different surface terminations. We then address the stability in electrochemical environments, and we investigate the OER mechanism, considering reaction paths involving both terminal and bridging oxygen atoms. We find that different surface reconstructions can promote OER via different reaction sites and potential-determining steps, albeit with a similar energy cost. In particular, the bulk-truncated termination promotes OER via terminal oxygen atoms, and the potential-determining step is the dehydrogenation of the *OH group. On the  R45° reconstruction, on the other hand, OER proceeds via the bridging oxygen atoms, and the potential-determining step is the formation of the hydroperoxo.
R45° reconstruction, on the other hand, OER proceeds via the bridging oxygen atoms, and the potential-determining step is the formation of the hydroperoxo.
中文翻译:

Fe3O4(001) 表面的析氧反应:对末端和桥接氧原子作用的理论见解
模型催化剂,其中具有明确定义的表面终止的单晶材料的结构可以在原子水平上确定,是进行电催化基础研究的有用系统。磁铁矿 Fe 3 O 4(001) 表面尤其是几项研究的重点,旨在表征其在存在水层的情况下在真空中的结构并与液态水基电解质接触。最近,该系统也被研究作为析氧反应 (OER) 的催化剂,目的是将界面的结构特性与催化性能和机理相关联。在这项工作中,我们使用基于密度泛函理论的第一性原理模拟来建立 Fe 3 O 4的结构、热力学和电子特性。(001) 表面与水接触。我们计算了磁铁矿/水系统的相图,并解决了在不同表面终端之间通过实验观察到的结构转变的一些悬而未决的问题。然后,我们解决了电化学环境中的稳定性问题,并研究了 OER 机制,同时考虑了涉及末端和桥接氧原子的反应路径。我们发现不同的表面重建可以通过不同的反应位点和决定电位的步骤促进 OER ,尽管具有相似的能量成本。特别是,体截断的末端通过末端氧原子促进 OER ,而决定电位的步骤是 *OH 基团的脱氢。在R 另一方面,45°重建通过桥接氧原子进行,而决定电位的步骤是氢过氧化物的形成。
另一方面,45°重建通过桥接氧原子进行,而决定电位的步骤是氢过氧化物的形成。
更新日期:2021-09-02
 R45° reconstruction, on the other hand, OER proceeds via the bridging oxygen atoms, and the potential-determining step is the formation of the hydroperoxo.
R45° reconstruction, on the other hand, OER proceeds via the bridging oxygen atoms, and the potential-determining step is the formation of the hydroperoxo.
中文翻译:

Fe3O4(001) 表面的析氧反应:对末端和桥接氧原子作用的理论见解
模型催化剂,其中具有明确定义的表面终止的单晶材料的结构可以在原子水平上确定,是进行电催化基础研究的有用系统。磁铁矿 Fe 3 O 4(001) 表面尤其是几项研究的重点,旨在表征其在存在水层的情况下在真空中的结构并与液态水基电解质接触。最近,该系统也被研究作为析氧反应 (OER) 的催化剂,目的是将界面的结构特性与催化性能和机理相关联。在这项工作中,我们使用基于密度泛函理论的第一性原理模拟来建立 Fe 3 O 4的结构、热力学和电子特性。(001) 表面与水接触。我们计算了磁铁矿/水系统的相图,并解决了在不同表面终端之间通过实验观察到的结构转变的一些悬而未决的问题。然后,我们解决了电化学环境中的稳定性问题,并研究了 OER 机制,同时考虑了涉及末端和桥接氧原子的反应路径。我们发现不同的表面重建可以通过不同的反应位点和决定电位的步骤促进 OER ,尽管具有相似的能量成本。特别是,体截断的末端通过末端氧原子促进 OER ,而决定电位的步骤是 *OH 基团的脱氢。在R
 另一方面,45°重建通过桥接氧原子进行,而决定电位的步骤是氢过氧化物的形成。
另一方面,45°重建通过桥接氧原子进行,而决定电位的步骤是氢过氧化物的形成。












































 京公网安备 11010802027423号
京公网安备 11010802027423号