Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2021-08-18 , DOI: 10.1016/j.jhazmat.2021.126943 A Mai 1 , E Hadnagy 2 , J Abraham 1 , A Terracciano 1 , Z Zheng 3 , B Smolinski 4 , A Koutsospyros 1 , C Christodoulatos 1
|
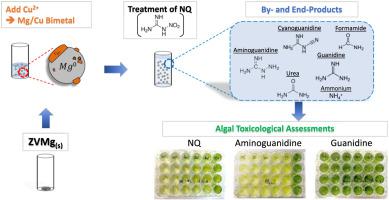
Energetic-laden process water from industrial munition facilities can be treated by zero-valent metals (ZVMs) or zero-valent iron (ZVI) to remove residual energetics. This reduction-based treatment is significantly enhanced with the addition of a secondary catalytic metal (i.e. forming a bimetal reagent). The reagent is further enhanced by using a more reductive base metal, such as Mg. In this work, the reductive degradation of nitroguanidine (NQ) in aqueous solutions by Mg/Cu bimetal is investigated. Two initial pH conditions (unadjusted and pH 2.7) were studied. Under unadjusted initial pH conditions, 90% of NQ degraded within 30 min reaction time. After 150 min, NQ degradation generated a suite of products including guanidine (44%), cyanamide (31%), formamide (15%), aminoguanidine (AQ) (6%), urea (2%) and cyanoguanidine (0.03%), leading to 100.0% carbon closure when accounting for residual NQ. The experimentally-derived degradation reaction pathway consisted of two parallel reactions: nitroreduction led to formation of AQ with further degradation to urea, cyanamide and formamide, or reductive cleavage of the N-N bond led to guanidine formation. Toxicological assessments indicated only cyanamide and AQ were toxic to S. obliquus at certain concentrations. A lowered initial pH promoted AQ transformation to benign formamide, thus reducing toxicity and complexity of products.
中文翻译:

测定镁基双金属 Mg/Cu 还原处理硝基胍 (NQ) 的降解动力学、副产物和毒性
来自工业弹药设施的充满能量的工艺水可以通过零价金属 (ZVM) 或零价铁 (ZVI) 进行处理,以去除残留的能量。这种基于还原的处理通过添加二次催化金属(即形成双金属试剂)得到显着增强。通过使用还原性更强的贱金属(例如 Mg)进一步增强了该试剂的性能。在这项工作中,研究了 Mg/Cu 双金属对水溶液中硝基胍 (NQ) 的还原降解。研究了两种初始 pH 条件(未调整和 pH 2.7)。在未调整的初始 pH 条件下,90% 的 NQ 在 30 分钟的反应时间内降解。150 分钟后,NQ 降解产生了一系列产物,包括胍 (44%)、氰胺 (31%)、甲酰胺 (15%)、氨基胍 (AQ) (6%)、尿素 (2%) 和氰基胍 (0.03%) ,导致 100。考虑到剩余 NQ 时,碳封闭为 0%。实验衍生的降解反应途径由两个平行反应组成:硝基还原导致形成 AQ,进一步降解为尿素、氰胺和甲酰胺,或 NN 键的还原裂解导致形成胍。毒理学评估表明只有氰胺和 AQ 对某些浓度下的S. obliquus。降低的初始 pH 值促进了 AQ 向良性甲酰胺的转化,从而降低了产品的毒性和复杂性。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号