Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unraveling the Synergy of Chemical Hydroxylation and the Physical Heterointerface upon Improving the Hydrogen Evolution Kinetics
ACS Nano ( IF 15.8 ) Pub Date : 2021-08-18 , DOI: 10.1021/acsnano.1c05324
Yang Liu 1, 2 , Xinghui Liu 1, 2 , Xiaoshan Wang 3 , Hui Ning 3 , Taehun Yang 1, 2 , Jianmin Yu 1, 2 , Ashwani Kumar 1, 2 , Yongguang Luo 1, 2 , Hongdan Wang 1, 2 , Lingling Wang 1 , Jinsun Lee 1, 2 , Amol R Jadhav 1 , Han Hu 3 , Mingbo Wu 3 , Min Gyu Kim 4 , Hyoyoung Lee 1, 2, 5, 6
ACS Nano ( IF 15.8 ) Pub Date : 2021-08-18 , DOI: 10.1021/acsnano.1c05324
Yang Liu 1, 2 , Xinghui Liu 1, 2 , Xiaoshan Wang 3 , Hui Ning 3 , Taehun Yang 1, 2 , Jianmin Yu 1, 2 , Ashwani Kumar 1, 2 , Yongguang Luo 1, 2 , Hongdan Wang 1, 2 , Lingling Wang 1 , Jinsun Lee 1, 2 , Amol R Jadhav 1 , Han Hu 3 , Mingbo Wu 3 , Min Gyu Kim 4 , Hyoyoung Lee 1, 2, 5, 6
Affiliation
|
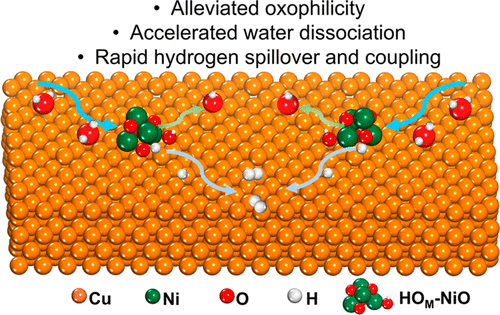
Efficient transition metal oxide electrocatalysts for the alkaline hydrogen evolution reaction (HER) have received intensive attention to energy conversion but are limited by their sluggish water dissociation and unfavorable hydrogen migration and coupling. Herein, systematic density functional theory (DFT) predicts that on representative NiO, the hydroxylation (OH–) and heterointerface coupled with metallic Cu can respectively reduce the energy barrier of water dissociation and facilitate hydrogen spillover. Motivated by theoretical predictions, we subtly designed a delicate strategy to realize the electrochemical OH– modification in KOH with moderate concentration (HOM-NiO) and to channel rapid hydrogen spillover at the heterointerface of HOM-NiO and Cu, ensuring an enhanced HER kinetic. This HOM-NiO/Cu is systematically investigated by in situ XAS and electrochemical simulations, verifying its extraordinary merits for HER including the enhanced water dissociation, alleviated oxophilicity that is advantageous for consecutive adsorptions of water, and accelerated hydrogen spillover, thereby exhibiting superb HER activity with 33 and 310 mV overpotentials at the current densities of 10 and 1000 mA cm–2 in 1.0 M KOH, outperforming the Pt/C. This study might provide a reasonable strategy for the functionalized design of superior electrocatalysts.
中文翻译:

揭示化学羟基化与物理异质界面在改善析氢动力学方面的协同作用
用于碱性析氢反应 (HER) 的高效过渡金属氧化物电催化剂在能量转换方面受到了广泛关注,但受到其缓慢的水解离和不利的氢迁移和耦合的限制。在此,系统密度泛函理论(DFT)预测,在代表性的 NiO 上,羟基化(OH -)和异质界面与金属 Cu 结合可以分别降低水解离的能垒并促进氢溢出。在理论预测的推动下,我们巧妙地设计了一种巧妙的策略来实现电化学 OH -在中等浓度的 KOH(H O M -NiO)中进行修饰,并在 H O M的异质界面处引导快速氢溢出-NiO 和 Cu,确保增强的 HER 动力学。通过原位XAS 和电化学模拟系统地研究了这种 HO M -NiO/Cu ,验证了其对 HER 的非凡优点,包括增强的水解离、减轻有利于连续吸附水的亲氧性和加速氢溢出,从而表现出卓越的 HER在 1.0 M KOH 中,在10 和 1000 mA cm –2的电流密度下具有 33 和 310 mV 的过电位,表现优于 Pt/C。该研究可能为优质电催化剂的功能化设计提供合理的策略。
更新日期:2021-09-28
中文翻译:

揭示化学羟基化与物理异质界面在改善析氢动力学方面的协同作用
用于碱性析氢反应 (HER) 的高效过渡金属氧化物电催化剂在能量转换方面受到了广泛关注,但受到其缓慢的水解离和不利的氢迁移和耦合的限制。在此,系统密度泛函理论(DFT)预测,在代表性的 NiO 上,羟基化(OH -)和异质界面与金属 Cu 结合可以分别降低水解离的能垒并促进氢溢出。在理论预测的推动下,我们巧妙地设计了一种巧妙的策略来实现电化学 OH -在中等浓度的 KOH(H O M -NiO)中进行修饰,并在 H O M的异质界面处引导快速氢溢出-NiO 和 Cu,确保增强的 HER 动力学。通过原位XAS 和电化学模拟系统地研究了这种 HO M -NiO/Cu ,验证了其对 HER 的非凡优点,包括增强的水解离、减轻有利于连续吸附水的亲氧性和加速氢溢出,从而表现出卓越的 HER在 1.0 M KOH 中,在10 和 1000 mA cm –2的电流密度下具有 33 和 310 mV 的过电位,表现优于 Pt/C。该研究可能为优质电催化剂的功能化设计提供合理的策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号