Synthesis ( IF 2.2 ) Pub Date : 2021-08-16 , DOI: 10.1055/s-0040-1706282 Alexander Shivanyuk 1, 2 , Alexey Chuyko 1 , Grygoriy Dolgonos 1 , Volodymyr Fetyukhin 1 , Oleg Lukin 1
|
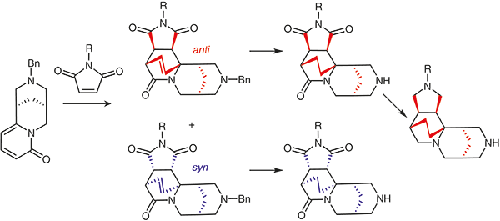
The Diels–Alder reaction of N-benzylcytisine with N-methyl- and N-benzylmaleimides is 100% endo-selective and gives the corresponding syn- and anti-diastereomers in 11–42% isolated yields. The studies of the reaction progress with LCMS and NMR along with detailed quantum chemical calculations revealed that some Diels–Alder adducts are kinetically and their isomers are thermodynamically controlled products. The Pd/C-catalyzed hydrogenation of benzyl-protected cytisine amine derivatives resulted in the removal of the benzyl group and the addition of hydrogen to the C=C double bond to give the corresponding secondary amines in 45–84% yield. The complete reduction of carbonyl groups in a cytisine derivative with LiAlH4 in THF under reflux afforded the respective tricyclic triamine. Quantum mechanical calculations for the mechanism of the Diels–Alder reaction between the simplest model compounds are presented.
中文翻译:

从 (-)-Cytisine 的 Diels-Alder 加合物简单合成复杂胺
N-苄基胞嘧啶与N-甲基-和N-苄基马来酰亚胺的 Diels-Alder 反应是 100%内选择性,并给出相应的顺-和反-非对映异构体的分离产率为 11-42%。用 LCMS 和 NMR 以及详细的量子化学计算对反应进程的研究表明,一些 Diels-Alder 加合物是动力学的,它们的异构体是热力学控制的产物。苄基保护的胞嘧啶胺衍生物的 Pd/C 催化氢化导致苄基的去除和氢加成到 C=C 双键,以 45-84% 的产率得到相应的仲胺。在回流下,在THF中用LiAlH 4完全还原胞苷衍生物中的羰基,得到相应的三环三胺。给出了最简单模型化合物之间 Diels-Alder 反应机理的量子力学计算。































 京公网安备 11010802027423号
京公网安备 11010802027423号