当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unified Approach to Benzo[d]thiazol-2-yl-Sulfonamides
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-08-16 , DOI: 10.1021/acs.joc.1c00317 František Zálešák 1 , Ondřej Kováč 1 , Eliška Lachetová 1 , Nikola Št'astná 1 , Jiří Pospíšil 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-08-16 , DOI: 10.1021/acs.joc.1c00317 František Zálešák 1 , Ondřej Kováč 1 , Eliška Lachetová 1 , Nikola Št'astná 1 , Jiří Pospíšil 1, 2
Affiliation
|
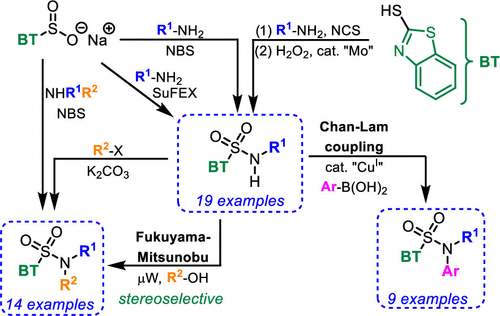
In this paper, we report a unified approach to N-substituted and N,N-disubstituted benzothiazole (BT) sulfonamides. Our approach to BT-sulfonamides starts from simple commercially available building blocks (benzo[d]thiazole-2-thiol and primary and secondary amines) that are connected via (a) a S oxidation/S–N coupling approach, (b) a S–N coupling/S-oxidation sequence, or via (c) a S-oxidation/S–F bond formation/SuFEx approach. The labile N–H bond in N-monoalkylated BT-sulfonamides (pKa (BTSO2N(H)Bn) = 3.34 ± 0.05) further allowed us to develop a simple weak base-promoted N-alkylation method and a stereoselective microwave-promoted Fukuyama–Mitsunobu reaction. N-Alkyl-N-aryl BT-sulfonamides were accessed with the help of the Chan–Lam coupling reaction. Developed methods were further used in stereo and chemoselective transformations of podophyllotoxin and several amino alcohols.
中文翻译:

苯并[d]噻唑-2-基-磺酰胺的统一方法
在本文中,我们报告了N-取代和N,N-二取代苯并噻唑 (BT) 磺酰胺的统一方法。我们的 BT-磺酰胺方法从简单的市售构件(苯并[ d ]噻唑-2-硫醇和伯胺和仲胺)开始,它们通过(a)S 氧化/S-N 偶联方法连接,(b)a S-N 偶联/S-氧化序列,或通过(c)S-氧化/S-F 键形成/SuFEx 方法。在不稳定的N-H键Ñ单烷基化BT-磺酰胺(对ķ一个(BTSO 2 N(ħ)Bn) = 3.34 ± 0.05) 进一步使我们能够开发一种简单的弱碱促进 N-烷基化方法和立体选择性微波促进 Fukuyama-Mitsunobu 反应。在 Chan-Lam 偶联反应的帮助下,获得了N-烷基-N-芳基 BT-磺酰胺。开发的方法进一步用于鬼臼毒素和几种氨基醇的立体和化学选择性转化。
更新日期:2021-09-03
中文翻译:

苯并[d]噻唑-2-基-磺酰胺的统一方法
在本文中,我们报告了N-取代和N,N-二取代苯并噻唑 (BT) 磺酰胺的统一方法。我们的 BT-磺酰胺方法从简单的市售构件(苯并[ d ]噻唑-2-硫醇和伯胺和仲胺)开始,它们通过(a)S 氧化/S-N 偶联方法连接,(b)a S-N 偶联/S-氧化序列,或通过(c)S-氧化/S-F 键形成/SuFEx 方法。在不稳定的N-H键Ñ单烷基化BT-磺酰胺(对ķ一个(BTSO 2 N(ħ)Bn) = 3.34 ± 0.05) 进一步使我们能够开发一种简单的弱碱促进 N-烷基化方法和立体选择性微波促进 Fukuyama-Mitsunobu 反应。在 Chan-Lam 偶联反应的帮助下,获得了N-烷基-N-芳基 BT-磺酰胺。开发的方法进一步用于鬼臼毒素和几种氨基醇的立体和化学选择性转化。































 京公网安备 11010802027423号
京公网安备 11010802027423号