当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Pd/Cu-Catalyzed Vinylation of Terminal Alkynes with (2-Bromoethyl)diphenylsulfonium Triflate
Organic Letters ( IF 4.9 ) Pub Date : 2021-08-16 , DOI: 10.1021/acs.orglett.1c02379 Xiao-Xia Ming 1 , Shuai Wu 1 , Ze-Yu Tian 1 , Jia-Wei Song 1 , Cheng-Pan Zhang 1
Organic Letters ( IF 4.9 ) Pub Date : 2021-08-16 , DOI: 10.1021/acs.orglett.1c02379 Xiao-Xia Ming 1 , Shuai Wu 1 , Ze-Yu Tian 1 , Jia-Wei Song 1 , Cheng-Pan Zhang 1
Affiliation
|
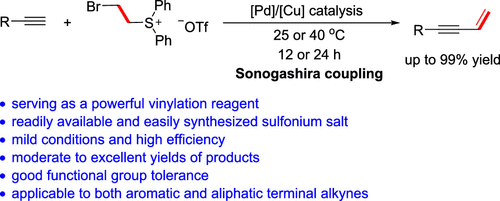
The potential of (2-bromoethyl)diphenylsulfonium triflate to be a powerful vinylation reagent was determined by the Sonogashira cross-coupling reactions with terminal alkynes. The vinylation proceeded smoothly at 25 °C under Pd/Cu catalysis to afford a variety of 1- and 2-unsubstituted 1,3-enynes in moderate to excellent yields. This protocol represents the first application of (2-haloethyl)diphenylsulfonium triflate as a CH═CH2 transfer source in organic synthesis.
中文翻译:

Pd/Cu 催化末端炔烃与(2-溴乙基)二苯基锍三氟甲磺酸盐的乙烯基化
(2-溴乙基)二苯基锍三氟甲磺酸盐作为强力乙烯基化试剂的潜力是通过与末端炔烃的 Sonogashira 交叉偶联反应确定的。在 Pd/Cu 催化下,乙烯基化在 25°C 下顺利进行,以中等至优异的产率提供各种 1-和 2-未取代的 1,3-烯炔。该方案代表了(2-卤乙基)二苯基锍三氟甲磺酸盐在有机合成中作为 CH=CH 2转移源的首次应用。
更新日期:2021-09-03
中文翻译:

Pd/Cu 催化末端炔烃与(2-溴乙基)二苯基锍三氟甲磺酸盐的乙烯基化
(2-溴乙基)二苯基锍三氟甲磺酸盐作为强力乙烯基化试剂的潜力是通过与末端炔烃的 Sonogashira 交叉偶联反应确定的。在 Pd/Cu 催化下,乙烯基化在 25°C 下顺利进行,以中等至优异的产率提供各种 1-和 2-未取代的 1,3-烯炔。该方案代表了(2-卤乙基)二苯基锍三氟甲磺酸盐在有机合成中作为 CH=CH 2转移源的首次应用。















































 京公网安备 11010802027423号
京公网安备 11010802027423号