Tetrahedron Letters ( IF 1.5 ) Pub Date : 2021-08-14 , DOI: 10.1016/j.tetlet.2021.153293 Lefeng Dong 1 , Junqing Liang 1 , Ruijia Chen 1 , Xiaoyong Xu 1 , Xusheng Shao 1 , Zhong Li 1
|
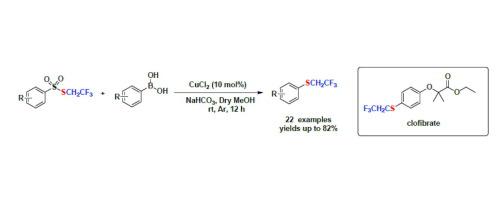
Trifluoroethylthio group (SCH2CF3) is a key functionality in several pharmaceutical and agrochemical compounds, the methods for the installation of the 2,2,2-trifluoroethylthio group are sought after. Herein, we report a copper-catalyzed trifluoroethylthiolation reaction of S-(2,2,2-trifluoroethyl) benzenesulfonothioates and phenylboronic acids at room temperature. The reaction achieved the insertion of trifluoroethylthio moiety to efficiently obtain various substituted aryl 2,2,2-trifluoroethyl thioethers in good yields. Mechanistic investigation indicates the trifluoroethylthiolation radical is involved in the catalytic circle. Moreover, trifluoroethylthiolated clofibrate was synthesized in a particular fashion.
中文翻译:

铜催化芳基硼酸与 PhSO2SCH2CF3 的自由基三氟乙基硫醇化反应
三氟乙硫基 (SCH 2 CF 3 ) 是几种药物和农用化学品中的关键官能团,安装 2,2,2-三氟乙硫基的方法受到追捧。在此,我们报告了铜催化的 S-(2,2,2-三氟乙基) 苯硫代苯磺酸盐和苯硼酸在室温下的三氟乙基硫醇化反应。该反应实现了三氟乙硫基的插入,以良好的收率有效地获得了各种取代的芳基2,2,2-三氟乙基硫醚。机理研究表明三氟乙基硫醇化自由基参与了催化循环。此外,以特定方式合成了三氟乙基硫醇化氯贝特。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号