当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Sweet H2S/H2O2 Dual Release System and Specific Protein S-Persulfidation Mediated by Thioglucose/Glucose Oxidase
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-08-12 , DOI: 10.1021/jacs.1c06372 Xiang Ni 1 , Xiaolu Li 2 , Tun-Li Shen 1 , Wei-Jun Qian 2 , Ming Xian 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-08-12 , DOI: 10.1021/jacs.1c06372 Xiang Ni 1 , Xiaolu Li 2 , Tun-Li Shen 1 , Wei-Jun Qian 2 , Ming Xian 1
Affiliation
|
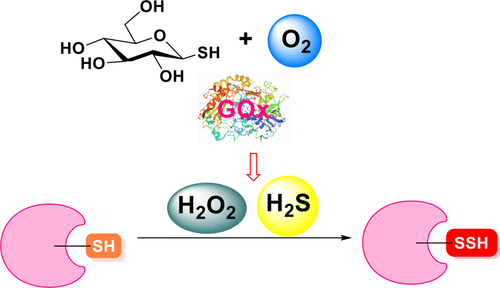
H2S and H2O2 are two redox regulating molecules that play important roles in many physiological and pathological processes. While each of them has distinct biosynthetic pathways and signaling mechanisms, the crosstalk between these two species is also known to cause critical biological responses such as protein S-persulfidation. So far, many chemical tools for the studies of H2S and H2O2 have been developed, such as the donors and sensors for H2S and H2O2. However, these tools are normally targeting single species (e.g., only H2S or only H2O2). As such, the crosstalk and synergetic effects between H2S and H2O2 have hardly been studied with those tools. In this work, we report a unique H2S/H2O2 dual donor system by employing 1-thio-β-d-glucose and glucose oxidase (GOx) as the substrates. This enzymatic system can simultaneously produce H2S and H2O2 in a slow and controllable fashion, without generating any bio-unfriendly byproducts. This system was demonstrated to cause efficient S-persulfidation on proteins. In addition, we expanded the system to thiolactose and thioglucose-disulfide; therefore, additional factors (β-galactosidase and cellular reductants) could be introduced to further control the release of H2S/H2O2. This dual release system should be useful for future research on H2S and H2O2.
中文翻译:

硫葡萄糖/葡萄糖氧化酶介导的甜 H2S/H2O2 双重释放系统和特异性蛋白质 S-过硫化
H 2 S 和 H 2 O 2是两种在许多生理和病理过程中发挥重要作用的氧化还原调节分子。虽然它们中的每一个都有不同的生物合成途径和信号传导机制,但已知这两个物种之间的串扰会引起关键的生物反应,例如蛋白质 S-过硫化。迄今为止,已经开发了许多用于研究H 2 S 和H 2 O 2的化学工具,例如H 2 S和H 2 O 2的供体和传感器。然而,这些工具通常针对单一物种(例如,仅 H 2 S 或仅 H 2 O 2)。因此,几乎没有使用这些工具研究H 2 S 和 H 2 O 2之间的串扰和协同效应。在这项工作中,我们报告了一种独特的 H 2 S/H 2 O 2双供体系统,采用 1-硫代-β- d-葡萄糖和葡萄糖氧化酶 (GOx) 作为底物。这种酶促系统可以同时产生H 2 S和H 2 O 2以缓慢且可控的方式,不会产生任何对生物不友好的副产品。该系统被证明可以对蛋白质产生有效的 S-过硫化。此外,我们将系统扩展到硫代乳糖和硫代葡萄糖二硫化物;因此,可以引入额外的因子(β-半乳糖苷酶和细胞还原剂)来进一步控制H 2 S/H 2 O 2的释放。这种双重释放系统应该对 H 2 S 和 H 2 O 2的未来研究有用。
更新日期:2021-08-25
中文翻译:

硫葡萄糖/葡萄糖氧化酶介导的甜 H2S/H2O2 双重释放系统和特异性蛋白质 S-过硫化
H 2 S 和 H 2 O 2是两种在许多生理和病理过程中发挥重要作用的氧化还原调节分子。虽然它们中的每一个都有不同的生物合成途径和信号传导机制,但已知这两个物种之间的串扰会引起关键的生物反应,例如蛋白质 S-过硫化。迄今为止,已经开发了许多用于研究H 2 S 和H 2 O 2的化学工具,例如H 2 S和H 2 O 2的供体和传感器。然而,这些工具通常针对单一物种(例如,仅 H 2 S 或仅 H 2 O 2)。因此,几乎没有使用这些工具研究H 2 S 和 H 2 O 2之间的串扰和协同效应。在这项工作中,我们报告了一种独特的 H 2 S/H 2 O 2双供体系统,采用 1-硫代-β- d-葡萄糖和葡萄糖氧化酶 (GOx) 作为底物。这种酶促系统可以同时产生H 2 S和H 2 O 2以缓慢且可控的方式,不会产生任何对生物不友好的副产品。该系统被证明可以对蛋白质产生有效的 S-过硫化。此外,我们将系统扩展到硫代乳糖和硫代葡萄糖二硫化物;因此,可以引入额外的因子(β-半乳糖苷酶和细胞还原剂)来进一步控制H 2 S/H 2 O 2的释放。这种双重释放系统应该对 H 2 S 和 H 2 O 2的未来研究有用。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号