当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Syn-Selective Nitroaldol Approach to Amphenicol Antibiotics: Evolution of a Unified Asymmetric Synthesis of (−)-Chloramphenicol, (−)-Azidamphenicol, (+)-Thiamphenicol, and (+)-Florfenicol
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-08-13 , DOI: 10.1021/acs.joc.1c01124 Yingqi Xia 1 , Meifen Jiang 2 , Minjie Liu 3 , Yan Zhang 1 , Hongmin Qu 1 , Tong Xiong 1 , Huashan Huang 3 , Dang Cheng 2 , Fener Chen 1, 2, 3
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-08-13 , DOI: 10.1021/acs.joc.1c01124 Yingqi Xia 1 , Meifen Jiang 2 , Minjie Liu 3 , Yan Zhang 1 , Hongmin Qu 1 , Tong Xiong 1 , Huashan Huang 3 , Dang Cheng 2 , Fener Chen 1, 2, 3
Affiliation
|
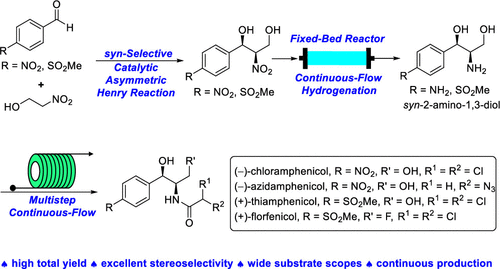
A unified strategy for an efficient and high diastereo- and enantioselective synthesis of (−)-chloramphenicol, (−)-azidamphenicol, (+)-thiamphenicol, and (+)-florfenicol based on a key catalytic syn-selective Henry reaction is reported. The stereochemistry of the ligand-enabled copper(II)-catalyzed aryl aldehyde Henry reaction of nitroethanol was first explored to forge a challenging syn-2-amino-1,3-diol structure unit with vicinal stereocenters with excellent stereocontrol. Multistep continuous flow manipulations were carried out to achieve the efficient asymmetric synthesis of this family of amphenicol antibiotics.
中文翻译:

氨苯尼考抗生素的催化合成选择性硝基羟醛方法:(-)-氯霉素、(-)-阿齐达芬尼、(+)-甲硫苯尼考和(+)-氟苯尼考的统一不对称合成的演变
报告了基于关键催化顺选择性亨利反应高效和高非对映选择性和对映选择性合成 (-)-氯霉素、(-)-阿齐丹苯、(+)-噻吩苯胺和 (+)-氟苯尼考的统一策略. 首次探索了配体启用的铜 (II) 催化的硝基乙醇的芳醛亨利反应的立体化学,以形成具有挑战性的合成-2-氨基-1,3-二醇结构单元,具有具有出色立体控制的邻位立体中心。进行了多步连续流动操作以实现该家族的双酚类抗生素的有效不对称合成。
更新日期:2021-09-03
中文翻译:

氨苯尼考抗生素的催化合成选择性硝基羟醛方法:(-)-氯霉素、(-)-阿齐达芬尼、(+)-甲硫苯尼考和(+)-氟苯尼考的统一不对称合成的演变
报告了基于关键催化顺选择性亨利反应高效和高非对映选择性和对映选择性合成 (-)-氯霉素、(-)-阿齐丹苯、(+)-噻吩苯胺和 (+)-氟苯尼考的统一策略. 首次探索了配体启用的铜 (II) 催化的硝基乙醇的芳醛亨利反应的立体化学,以形成具有挑战性的合成-2-氨基-1,3-二醇结构单元,具有具有出色立体控制的邻位立体中心。进行了多步连续流动操作以实现该家族的双酚类抗生素的有效不对称合成。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号