当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Selection of Macrocyclic Peptides That Bind STING From an mRNA-Display Library With Split Degenerate Codons
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-08-12 , DOI: 10.1002/anie.202103043
Chi-Wang Lin 1 , Mary J Harner 2 , Andrew E Douglas 2 , Virginie Lafont 2 , Fei Yu 2 , Ving G Lee 2 , Michael A Poss 2 , Joanna F Swain 3 , Martin Wright 1 , Daša Lipovšek 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-08-12 , DOI: 10.1002/anie.202103043
Chi-Wang Lin 1 , Mary J Harner 2 , Andrew E Douglas 2 , Virginie Lafont 2 , Fei Yu 2 , Ving G Lee 2 , Michael A Poss 2 , Joanna F Swain 3 , Martin Wright 1 , Daša Lipovšek 1
Affiliation
|
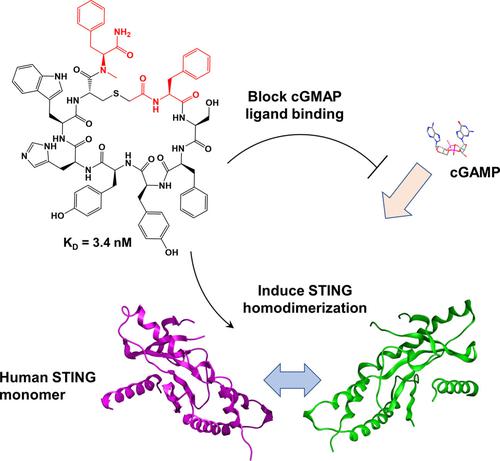
Recent improvements in mRNA display have enabled the selection of peptides that incorporate non-natural amino acids, thus expanding the chemical diversity of macrocycles beyond what is accessible in nature. Such libraries have incorporated non-natural amino acids at the expense of natural amino acids by reassigning their codons. Here we report an alternative approach to expanded amino-acid diversity that preserves all 19 natural amino acids (no methionine) and adds 6 non-natural amino acids, resulting in the highest sequence complexity reported to date. We have applied mRNA display to this 25-letter library to select functional macrocycles that bind human STING, a protein involved in immunoregulation. The resulting STING-binding peptides include a 9-mer macrocycle with a dissociation constant (KD) of 3.4 nM, which blocks binding of cGAMP to STING and induces STING dimerization. This approach is generalizable to expanding the amino-acid alphabet in a library beyond 25 building blocks.
中文翻译:

从具有分裂简并密码子的 mRNA 展示库中结合 STING 的大环肽的选择
mRNA 展示的最新改进使得能够选择包含非天然氨基酸的肽,从而将大环化合物的化学多样性扩大到自然界中无法获得的范围。此类文库通过重新分配密码子以牺牲天然氨基酸为代价掺入了非天然氨基酸。在这里,我们报告了一种扩展氨基酸多样性的替代方法,该方法保留了所有 19 种天然氨基酸(无蛋氨酸)并添加了 6 种非天然氨基酸,从而获得了迄今为止报道的最高序列复杂性。我们已将 mRNA 展示应用于这个 25 个字母的文库,以选择与人类 STING(一种参与免疫调节的蛋白质)结合的功能性大环化合物。由此产生的 STING 结合肽包括一个具有解离常数 ( K D) 的 3.4 nM,可阻断 cGAMP 与 STING 的结合并诱导 STING 二聚化。这种方法可推广到将库中的氨基酸字母表扩展到超过 25 个构建块。
更新日期:2021-10-04
中文翻译:

从具有分裂简并密码子的 mRNA 展示库中结合 STING 的大环肽的选择
mRNA 展示的最新改进使得能够选择包含非天然氨基酸的肽,从而将大环化合物的化学多样性扩大到自然界中无法获得的范围。此类文库通过重新分配密码子以牺牲天然氨基酸为代价掺入了非天然氨基酸。在这里,我们报告了一种扩展氨基酸多样性的替代方法,该方法保留了所有 19 种天然氨基酸(无蛋氨酸)并添加了 6 种非天然氨基酸,从而获得了迄今为止报道的最高序列复杂性。我们已将 mRNA 展示应用于这个 25 个字母的文库,以选择与人类 STING(一种参与免疫调节的蛋白质)结合的功能性大环化合物。由此产生的 STING 结合肽包括一个具有解离常数 ( K D) 的 3.4 nM,可阻断 cGAMP 与 STING 的结合并诱导 STING 二聚化。这种方法可推广到将库中的氨基酸字母表扩展到超过 25 个构建块。































 京公网安备 11010802027423号
京公网安备 11010802027423号