Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dopamine Receptor-Mediated Binding and Cellular Uptake of Polydopamine-Coated Nanoparticles
ACS Nano ( IF 15.8 ) Pub Date : 2021-08-11 , DOI: 10.1021/acsnano.1c06081 Yao Liu , Chun Kit K. Choi , Huiling Hong , Yu Xiao , Man Long Kwok , Hanzhuang Liu , Xiao Yu Tian , Chung Hang Jonathan Choi
ACS Nano ( IF 15.8 ) Pub Date : 2021-08-11 , DOI: 10.1021/acsnano.1c06081 Yao Liu , Chun Kit K. Choi , Huiling Hong , Yu Xiao , Man Long Kwok , Hanzhuang Liu , Xiao Yu Tian , Chung Hang Jonathan Choi
|
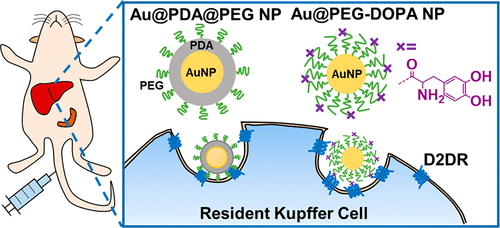
Polydopamine (PDA)-coated nanoparticles (NPs) are emerging carriers of therapeutic agents for nanomedicine applications due to their biocompatibility and abundant entry to various cell types, yet it remains unknown whether their cellular entry engages cell-surface receptors. As monomeric dopamine (DA) is an endogenous ligand of dopamine receptor and raw ingredient of PDA, we elucidate the interaction between polyethylene glycol-stabilized, PDA-coated gold NPs (Au@PDA@PEG NPs) and dopamine receptors, particularly D2 (D2DR). After proving the binding of Au@PDA@PEG NPs to recombinant and cellular D2DR, we employ antibody blocking, gene knockdown, and gene overexpression to establish the role of D2DR in the cellular uptake of Au@PDA@PEG NPs in vitro. By preparing a series of PEG-coated AuNPs that contain different structural analogues of DA (Au@PEG-X NPs), we demonstrate that catechol and amine groups collectively enhance the binding of NPs to D2DR and their cellular uptake. By intravenously injecting Au@PDA@PEG NPs to Balb/c mice, we reveal their in vivo binding to D2DR in the liver by competitive inhibition and immunohistochemistry together with their preferential association to D2DR-rich resident Kupffer cells by flow cytometry, a result consistent with the profuse expression of D2DR by resident Kupffer cells. Catechol and amine groups jointly contribute to the preferential association of NPs to D2DR-rich Kupffer cells. Our data highlight the importance of D2DR expression and DA-related functional groups in mediating the cell–nano interactions of PDA-based nanomedicines.
中文翻译:

多巴胺受体介导的聚多巴胺涂层纳米粒子的结合和细胞摄取
聚多巴胺 (PDA) 包被的纳米颗粒 (NP) 由于其生物相容性和大量进入各种细胞类型而成为纳米医学应用治疗剂的新兴载体,但它们的细胞进入是否与细胞表面受体结合仍然未知。由于单体多巴胺 (DA) 是多巴胺受体的内源性配体和 PDA 的原料,我们阐明了聚乙二醇稳定的、PDA 包被的金 NPs (Au@PDA@PEG NPs) 与多巴胺受体,特别是 D2 (D2DR) 之间的相互作用). 在证明 Au@PDA@PEG NPs 与重组和细胞 D2DR 的结合后,我们采用抗体阻断、基因敲低和基因过表达来确定 D2DR 在 Au@PDA@PEG NPs体外细胞摄取中的作用. 通过制备一系列含有不同 DA 结构类似物 (Au@PEG-X NPs) 的 PEG 涂层 AuNPs,我们证明儿茶酚和胺基团共同增强了 NPs 与 D2DR 的结合及其细胞摄取。通过向 Balb/c 小鼠静脉内注射 Au@PDA@PEG NPs,我们揭示了它们的体内通过竞争性抑制和免疫组织化学与肝脏中的 D2DR 结合,以及它们通过流式细胞术优先结合富含 D2DR 的常驻 Kupffer 细胞,这一结果与常驻 Kupffer 细胞大量表达 D2DR 一致。儿茶酚和胺基团共同促进 NP 与富含 D2DR 的枯否细胞的优先结合。我们的数据强调了 D2DR 表达和 DA 相关官能团在介导基于 PDA 的纳米药物的细胞-纳米相互作用中的重要性。
更新日期:2021-08-24
中文翻译:

多巴胺受体介导的聚多巴胺涂层纳米粒子的结合和细胞摄取
聚多巴胺 (PDA) 包被的纳米颗粒 (NP) 由于其生物相容性和大量进入各种细胞类型而成为纳米医学应用治疗剂的新兴载体,但它们的细胞进入是否与细胞表面受体结合仍然未知。由于单体多巴胺 (DA) 是多巴胺受体的内源性配体和 PDA 的原料,我们阐明了聚乙二醇稳定的、PDA 包被的金 NPs (Au@PDA@PEG NPs) 与多巴胺受体,特别是 D2 (D2DR) 之间的相互作用). 在证明 Au@PDA@PEG NPs 与重组和细胞 D2DR 的结合后,我们采用抗体阻断、基因敲低和基因过表达来确定 D2DR 在 Au@PDA@PEG NPs体外细胞摄取中的作用. 通过制备一系列含有不同 DA 结构类似物 (Au@PEG-X NPs) 的 PEG 涂层 AuNPs,我们证明儿茶酚和胺基团共同增强了 NPs 与 D2DR 的结合及其细胞摄取。通过向 Balb/c 小鼠静脉内注射 Au@PDA@PEG NPs,我们揭示了它们的体内通过竞争性抑制和免疫组织化学与肝脏中的 D2DR 结合,以及它们通过流式细胞术优先结合富含 D2DR 的常驻 Kupffer 细胞,这一结果与常驻 Kupffer 细胞大量表达 D2DR 一致。儿茶酚和胺基团共同促进 NP 与富含 D2DR 的枯否细胞的优先结合。我们的数据强调了 D2DR 表达和 DA 相关官能团在介导基于 PDA 的纳米药物的细胞-纳米相互作用中的重要性。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号