当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unraveling the Histidine Tautomerism Effect on the Initial Stages of Prion Misfolding: New Insights from a Computational Perspective
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2021-08-12 , DOI: 10.1021/acschemneuro.1c00376
Sompriya Chatterjee 1 , Abbas Salimi 1 , Jin Yong Lee 1
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2021-08-12 , DOI: 10.1021/acschemneuro.1c00376
Sompriya Chatterjee 1 , Abbas Salimi 1 , Jin Yong Lee 1
Affiliation
|
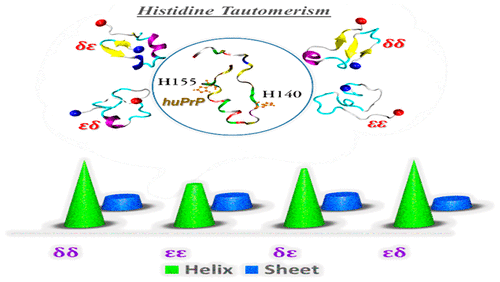
The aggregation and structural conversion of normal prion peptide (PrPC) into the pathogenic scrapie form (PrPSc), which can act as a seed to enhance prion amyloid fiber formation, is believed to be a crucial event in prionopathies. Previous research suggests that the prion monomer may play an important role in oligomer generation during disease pathogenesis. In the present study, extensive replica-exchange molecular dynamics (REMD) simulations were conducted to explore the conformational characteristics of the huPrP (125–160) monomer under the histidine tautomerism effect. Investigating the structural characteristics and fibrilization process is challenging because two histidine tautomers [Nε2-H (ε) and Nδ1-H (δ)] can occur in the open neutral state. Molecular dynamics (MD) simulation outcomes have shown that the toxic εδ and δδ isomer (containing several and broader local minima) had the highest α-helix structures, with contents of 21.11% and 21.01%, respectively, and may have a strong influence on the organizational behavior of a monomeric prion. The amino acids aspartate 20 (D20)–asparagine 29 (N29) and isoleucine 15 (I15)–histidine 16 (H16), D20–arginine 27 (R27) as well as N29 formed α-helix with the highest probabilities in the δδ and εδ isomer, accordingly. On the basis of our findings, we propose the histidine tautomerization hypothesis as a new prion accumulation mechanism, which may exist to induce the formation of prion accumulates. Overall, our tautomerism hypothesis constitutes a promising perspective for enhancing understanding of prion disease pathobiology and may help in the design of a good inhibitor.
中文翻译:

揭示组氨酸互变异构对朊病毒错误折叠初始阶段的影响:从计算角度的新见解
正常朊病毒肽 (PrP C )的聚集和结构转化为致病性痒病形式 (PrP Sc ),其可作为增强朊病毒淀粉样纤维形成的种子,被认为是朊病毒病中的关键事件。先前的研究表明,朊病毒单体可能在疾病发病过程中的寡聚体生成中发挥重要作用。在本研究中,进行了广泛的复制交换分子动力学 (REMD) 模拟,以探索组氨酸互变异构效应下 huPrP (125-160) 单体的构象特征。研究结构特征和纤维化过程具有挑战性,因为两个组氨酸互变异构体 [N ε2 -H (ε) 和 N δ1-H (δ)] 可以出现在开路中性状态。分子动力学 (MD) 模拟结果表明,有毒的 εδ 和 δδ 异构体(包含几个和更广泛的局部最小值)具有最高的 α-螺旋结构,含量分别为 21.11% 和 21.01%,可能对单体朊病毒的组织行为。氨基酸天冬氨酸 20 (D20)-天冬酰胺 29 (N29) 和异亮氨酸 15 (I15)-组氨酸 16 (H16)、D20-精氨酸 27 (R27) 以及 N29 形成 α-螺旋,在 δδ 和εδ 异构体,因此。基于我们的研究结果,我们提出组氨酸互变异构化假说作为一种新的朊病毒积累机制,可能存在诱导朊病毒积累的形成。总体,
更新日期:2021-09-01
中文翻译:

揭示组氨酸互变异构对朊病毒错误折叠初始阶段的影响:从计算角度的新见解
正常朊病毒肽 (PrP C )的聚集和结构转化为致病性痒病形式 (PrP Sc ),其可作为增强朊病毒淀粉样纤维形成的种子,被认为是朊病毒病中的关键事件。先前的研究表明,朊病毒单体可能在疾病发病过程中的寡聚体生成中发挥重要作用。在本研究中,进行了广泛的复制交换分子动力学 (REMD) 模拟,以探索组氨酸互变异构效应下 huPrP (125-160) 单体的构象特征。研究结构特征和纤维化过程具有挑战性,因为两个组氨酸互变异构体 [N ε2 -H (ε) 和 N δ1-H (δ)] 可以出现在开路中性状态。分子动力学 (MD) 模拟结果表明,有毒的 εδ 和 δδ 异构体(包含几个和更广泛的局部最小值)具有最高的 α-螺旋结构,含量分别为 21.11% 和 21.01%,可能对单体朊病毒的组织行为。氨基酸天冬氨酸 20 (D20)-天冬酰胺 29 (N29) 和异亮氨酸 15 (I15)-组氨酸 16 (H16)、D20-精氨酸 27 (R27) 以及 N29 形成 α-螺旋,在 δδ 和εδ 异构体,因此。基于我们的研究结果,我们提出组氨酸互变异构化假说作为一种新的朊病毒积累机制,可能存在诱导朊病毒积累的形成。总体,































 京公网安备 11010802027423号
京公网安备 11010802027423号