当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Cu2O Nanostructures with Tunable Crystal Facets for Electrochemical CO2 Reduction to Alcohols
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-08-12 , DOI: 10.1021/acsami.1c03850 Bingqian Liu 1 , Xi Yao 1 , Zijing Zhang 1 , Changhai Li 2 , Jiaqing Zhang 3 , Puyao Wang 1 , Jiayi Zhao 1 , Yafei Guo 1 , Jian Sun 1 , Chuanwen Zhao 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-08-12 , DOI: 10.1021/acsami.1c03850 Bingqian Liu 1 , Xi Yao 1 , Zijing Zhang 1 , Changhai Li 2 , Jiaqing Zhang 3 , Puyao Wang 1 , Jiayi Zhao 1 , Yafei Guo 1 , Jian Sun 1 , Chuanwen Zhao 1
Affiliation
|
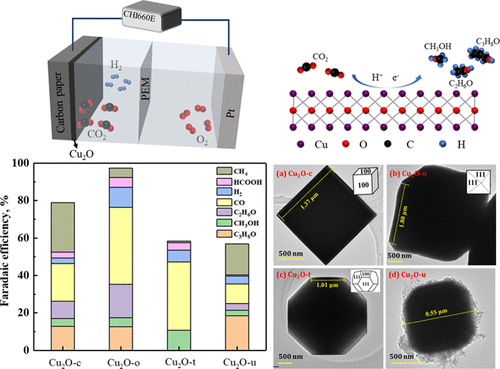
Electrochemical CO2 reduction enables the conversion of intermittent renewable energy to value-added chemicals and fuel, presenting a promising strategy to relieve CO2 emission and achieve clean energy storage. In this work, we developed nanosized Cu2O catalysts using the hydrothermal method for electrochemical CO2 reduction to alcohols. Cu2O nanoparticles (NPs) of various morphologies that were enclosed with different crystal facets, named as Cu2O-c (cubic structure with (100) facets), Cu2O-o (octahedron structure with (111) facets), Cu2O-t (truncated octahedron structure with both (100) and (111) facets), and Cu2O-u (urchin-like structure with (100), (220), and (222) facets), were prepared by regulating the content of a polyvinyl pyrrolidone (PVP) template. The electrochemical CO2 reduction performance of the different Cu2O NPs was evaluated in the CO2-saturated 0.5 M KHCO3 electrolyte. The as-synthesized Cu2O nanostructures were capable of reducing CO2 to produce alcohols including methanol, ethanol, and isopropanol. The alcohol selectivity of the different Cu2O NPs followed the order of Cu2O-t < Cu2O-u < Cu2O-c < Cu2O-o (with the total Faradaic efficiencies of alcohol products of 10.7, 25.0, 26.2, and 35.4%). The facet-dependent effects were associated with the varied concentrations of oxygen-vacancy defects, different energy barriers of CO2 reduction, and distinct Cu–O bond lengths over the different crystal facets. The desired Cu2O-o catalyst exhibited good reduction activity with the highest partial current density of 0.51 mA/cm2 for alcohols. The Faradaic efficiencies of alcohol products were 4.9% for methanol, 17.9% for ethanol, and 12.6% for isopropanol. The good electrochemical CO2 reduction performance was also associated with the surface reconstruction of Cu2O, which endowed the catalyst with abundant Cu0 and Cu+ sites for promoted CO2 activation and stabilized CO* adsorption for enhanced C–C coupling. This work will provide a new route for enhancing the alcohol selectivity of nanostructured Cu2O catalysts by crystal facet engineering.
中文翻译:

用于电化学 CO2 还原为醇的具有可调晶面的 Cu2O 纳米结构的合成
电化学CO 2还原能够将间歇性可再生能源转化为增值化学品和燃料,为减少CO 2排放和实现清洁能源储存提供了一种有前景的策略。在这项工作中,我们使用水热法开发了纳米尺寸的 Cu 2 O 催化剂,用于电化学 CO 2还原为醇。被不同晶面包围的各种形态的Cu 2 O纳米颗粒(NPs),命名为Cu 2 O-c(具有(100)个晶面的立方结构)、Cu 2 O-o(具有(111)个晶面的八面体结构)、Cu 2 Ot(具有 (100) 和 (111) 面)和 Cu 2 的截断八面体结构Ou(具有(100)、(220)和(222)面的海胆状结构)是通过调节聚乙烯吡咯烷酮(PVP)模板的含量制备的。在CO 2饱和的0.5 M KHCO 3电解质中评估了不同Cu 2 O NPs的电化学CO 2还原性能。合成的Cu 2 O 纳米结构能够还原CO 2以产生醇,包括甲醇、乙醇和异丙醇。不同Cu 2 O NPs的醇选择性顺序为Cu 2 O-t < Cu 2 O-u < Cu 2 O-c < Cu 2Oo(酒精产品的总法拉第效率为 10.7、25.0、26.2 和 35.4%)。晶面相关效应与不同浓度的氧空位缺陷、不同的 CO 2还原能垒以及不同晶面上不同的 Cu-O 键长有关。所需的Cu 2 O-o 催化剂表现出良好的还原活性,对醇类的最高分电流密度为0.51 mA/cm 2。酒精产品的法拉第效率为甲醇 4.9%、乙醇 17.9% 和异丙醇 12.6%。良好的电化学 CO 2还原性能还与 Cu 2 O的表面重构有关,这赋予了催化剂丰富的 Cu0和 Cu +位点用于促进 CO 2活化和稳定 CO* 吸附以增强 C-C 耦合。该工作将为通过晶面工程提高纳米结构Cu 2 O催化剂的醇选择性提供一条新途径。
更新日期:2021-08-25
中文翻译:

用于电化学 CO2 还原为醇的具有可调晶面的 Cu2O 纳米结构的合成
电化学CO 2还原能够将间歇性可再生能源转化为增值化学品和燃料,为减少CO 2排放和实现清洁能源储存提供了一种有前景的策略。在这项工作中,我们使用水热法开发了纳米尺寸的 Cu 2 O 催化剂,用于电化学 CO 2还原为醇。被不同晶面包围的各种形态的Cu 2 O纳米颗粒(NPs),命名为Cu 2 O-c(具有(100)个晶面的立方结构)、Cu 2 O-o(具有(111)个晶面的八面体结构)、Cu 2 Ot(具有 (100) 和 (111) 面)和 Cu 2 的截断八面体结构Ou(具有(100)、(220)和(222)面的海胆状结构)是通过调节聚乙烯吡咯烷酮(PVP)模板的含量制备的。在CO 2饱和的0.5 M KHCO 3电解质中评估了不同Cu 2 O NPs的电化学CO 2还原性能。合成的Cu 2 O 纳米结构能够还原CO 2以产生醇,包括甲醇、乙醇和异丙醇。不同Cu 2 O NPs的醇选择性顺序为Cu 2 O-t < Cu 2 O-u < Cu 2 O-c < Cu 2Oo(酒精产品的总法拉第效率为 10.7、25.0、26.2 和 35.4%)。晶面相关效应与不同浓度的氧空位缺陷、不同的 CO 2还原能垒以及不同晶面上不同的 Cu-O 键长有关。所需的Cu 2 O-o 催化剂表现出良好的还原活性,对醇类的最高分电流密度为0.51 mA/cm 2。酒精产品的法拉第效率为甲醇 4.9%、乙醇 17.9% 和异丙醇 12.6%。良好的电化学 CO 2还原性能还与 Cu 2 O的表面重构有关,这赋予了催化剂丰富的 Cu0和 Cu +位点用于促进 CO 2活化和稳定 CO* 吸附以增强 C-C 耦合。该工作将为通过晶面工程提高纳米结构Cu 2 O催化剂的醇选择性提供一条新途径。































 京公网安备 11010802027423号
京公网安备 11010802027423号