当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Self-Activated Ni Cathode for Electrocatalytic Nitrate Reduction to Ammonia: From Fundamentals to Scale-Up for Treatment of Industrial Wastewater
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-08-11 , DOI: 10.1021/acs.est.1c02278 Wenxiao Zheng 1 , Liuyi Zhu 1 , Zhang Yan 1 , Zichao Lin 1 , Zhenchao Lei 1 , Yifan Zhang 1 , Haolin Xu 1 , Zhi Dang 1 , Chaohai Wei 1 , Chunhua Feng 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-08-11 , DOI: 10.1021/acs.est.1c02278 Wenxiao Zheng 1 , Liuyi Zhu 1 , Zhang Yan 1 , Zichao Lin 1 , Zhenchao Lei 1 , Yifan Zhang 1 , Haolin Xu 1 , Zhi Dang 1 , Chaohai Wei 1 , Chunhua Feng 1
Affiliation
|
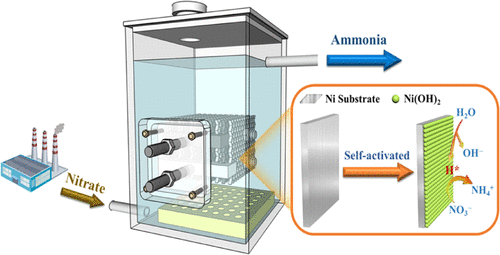
Electrocatalytic reduction has recently received increasing attention as a method of converting waste nitrate into value-added ammonia, but most studies have focused on complex strategies of catalyst preparation and little has been done in the way of large-scale demonstrations. Herein, we report that in situ activation of a pristine Ni electrode, either on a lab scale or a pilot scale, is effective in facilitating nitrate reduction to ammonia, exhibiting extraordinarily high activity, selectivity, and stability. The self-activated Ni cathode has a robust capacity to reduce nitrate over a wide range of concentrations and achieves great conversion yield, NH4+–N selectivity, and Faradaic efficiency, respectively, 95.3, 95.5, and 64.4% at 200 mg L–1 NO3––N and 97.8, 97.1, and 90.4% at 2000 mg L–1 NO3––N, for example. Fundamental research indicates that Ni(OH)2 nanoparticles are formed on the Ni electrode surface upon self-activation, which play crucial roles in governing nitrate reduction reaction (NO3RR) through the atomic H*-mediated pathway and accordingly suppressing hydrogen evolution reaction. More importantly, the self-activated Ni(OH)2@Ni cathode can be easily scaled up to allow large volumes of real industrial wastewater to be processed, successfully transferring nitrate into ammonia with high yields and Faradaic efficiency. This study demonstrates a new, mild, and promising method of cleaning nitrate-laden wastewater that produces ammonia as a valuable byproduct.
中文翻译:

用于电催化硝酸盐还原为氨的自活化镍阴极:从基础到放大用于处理工业废水
电催化还原作为一种将废硝酸盐转化为具有附加值的氨的方法最近受到越来越多的关注,但大多数研究都集中在催化剂制备的复杂策略上,而在大规模示范方面做得很少。在此,我们报告了在实验室规模或中试规模上原位活化原始 Ni 电极可有效促进硝酸盐还原为氨,表现出极高的活性、选择性和稳定性。自活化镍阴极具有强大的还原能力,可以在很宽的浓度范围内还原硝酸盐,并实现了很高的转化率、NH 4 + –N 选择性和法拉第效率,在 200 mg L – 时分别为 95.3、95.5 和 64.4%。1没有3– –N 和 97.8、97.1 和 90.4% 在 2000 mg L –1 NO 3 – –N,例如。基础研究表明,Ni(OH) 2纳米粒子通过自激活在Ni电极表面形成,在通过原子H*介导的途径控制硝酸盐还原反应(NO 3 RR)并因此抑制析氢反应中起关键作用. 更重要的是,自激活的 Ni(OH) 2@Ni 阴极可以轻松放大以处理大量实际工业废水,成功地将硝酸盐转化为高产率和法拉第效率的氨。这项研究展示了一种新的、温和的、有前途的方法,用于清洁富含硝酸盐的废水,该废水会产生氨作为有价值的副产品。
更新日期:2021-10-06
中文翻译:

用于电催化硝酸盐还原为氨的自活化镍阴极:从基础到放大用于处理工业废水
电催化还原作为一种将废硝酸盐转化为具有附加值的氨的方法最近受到越来越多的关注,但大多数研究都集中在催化剂制备的复杂策略上,而在大规模示范方面做得很少。在此,我们报告了在实验室规模或中试规模上原位活化原始 Ni 电极可有效促进硝酸盐还原为氨,表现出极高的活性、选择性和稳定性。自活化镍阴极具有强大的还原能力,可以在很宽的浓度范围内还原硝酸盐,并实现了很高的转化率、NH 4 + –N 选择性和法拉第效率,在 200 mg L – 时分别为 95.3、95.5 和 64.4%。1没有3– –N 和 97.8、97.1 和 90.4% 在 2000 mg L –1 NO 3 – –N,例如。基础研究表明,Ni(OH) 2纳米粒子通过自激活在Ni电极表面形成,在通过原子H*介导的途径控制硝酸盐还原反应(NO 3 RR)并因此抑制析氢反应中起关键作用. 更重要的是,自激活的 Ni(OH) 2@Ni 阴极可以轻松放大以处理大量实际工业废水,成功地将硝酸盐转化为高产率和法拉第效率的氨。这项研究展示了一种新的、温和的、有前途的方法,用于清洁富含硝酸盐的废水,该废水会产生氨作为有价值的副产品。

































 京公网安备 11010802027423号
京公网安备 11010802027423号