当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, and Insecticidal Activity of Novel Triazone Derivatives Containing Sulfonamide or Sulfonimide Moieties
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2021-08-10 , DOI: 10.1021/acs.jafc.1c01925 Yan Yang 1, 2 , Yuxiu Liu 1 , Hongjian Song 1 , Yongqiang Li 1 , Qingmin Wang 1
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2021-08-10 , DOI: 10.1021/acs.jafc.1c01925 Yan Yang 1, 2 , Yuxiu Liu 1 , Hongjian Song 1 , Yongqiang Li 1 , Qingmin Wang 1
Affiliation
|
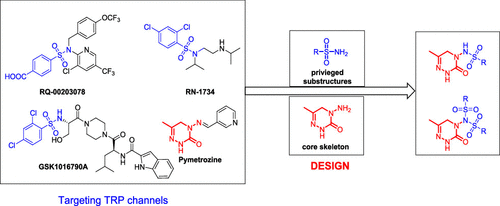
On the basis of sulfonamide moieties widely used in drugs and the mode of action of pymetrozine and the structures of some transient receptor potential channel antagonists and to improve the insecticidal activities of triazone insecticides, a series of triazone derivatives containing sulfonamide or sulfonimide moieties were designed, synthesized, and characterized. The bioassay results showed that most of these derivatives containing sulfonamide moieties exhibited excellent insecticidal activities against Aphis craccivora. Especially, (substituted)phenylsulfonylamino triazones I-9 (LC50 = 2.9869 mg/kg), I-10 (LC50 = 3.4957 mg/kg), I-11 (LC50 = 2.3154 mg/kg), I-12 (LC50 = 4.1614 mg/kg), I-13 (LC50 = 2.1690 mg/kg), and I-14 (LC50 = 3.0077 mg/kg) exhibited much higher aphicidal activity than pymetrozine (LC50 = 6.0635 mg/kg). All monosulfonyl-substituted 4-amino-triazinones had better activities than the corresponding disulfonyl-substituted compounds. In addition, some derivatives also exhibited insecticidal activities against Culex pipiens pallens. Among Helicoverpa armigera, Mythimna separata, and Ostrinia nubilalis, compounds I-18 and I-21 exhibited good larvicidal activities (LC50 values were 0.6929 and 0.2592 mg/kg, respectively) against C. pipiens pallens.
中文翻译:

含有磺酰胺或磺酰亚胺部分的新型三唑酮衍生物的设计、合成和杀虫活性
基于药物中广泛使用的磺酰胺基团和吡甲肼的作用方式和一些瞬时受体电位通道拮抗剂的结构,为提高三唑酮类杀虫剂的杀虫活性,设计了一系列含有磺胺或磺酰亚胺基团的三唑酮衍生物,合成,表征。生物测定结果表明,这些含有磺酰胺基团的衍生物大多对蚜虫表现出优异的杀虫活性。特别是,(取代的)苯磺酰氨基三氮酮I-9 (LC 50 = 2.9869 mg/kg)、I-10 (LC 50 = 3.4957 mg/kg)、I-11 (LC 50= 2.3154 mg/kg)、I-12 (LC 50 = 4.1614 mg/kg)、I-13 (LC 50 = 2.1690 mg/kg) 和I-14 (LC 50 = 3.0077 mg/kg) 表现出更高的杀蚜性活性高于吡蚜酮 (LC 50 = 6.0635 mg/kg)。所有单磺酰基取代的 4-氨基三嗪酮都比相应的二磺酰基取代化合物具有更好的活性。此外,一些衍生物还表现出对淡色库蚊的杀虫活性。间棉铃虫,粘虫,和欧洲玉米螟,化合物I-18和I-21对C. pipiens pallens表现出良好的杀幼虫活性(LC 50值分别为 0.6929 和 0.2592 mg/kg)。
更新日期:2021-09-22
中文翻译:

含有磺酰胺或磺酰亚胺部分的新型三唑酮衍生物的设计、合成和杀虫活性
基于药物中广泛使用的磺酰胺基团和吡甲肼的作用方式和一些瞬时受体电位通道拮抗剂的结构,为提高三唑酮类杀虫剂的杀虫活性,设计了一系列含有磺胺或磺酰亚胺基团的三唑酮衍生物,合成,表征。生物测定结果表明,这些含有磺酰胺基团的衍生物大多对蚜虫表现出优异的杀虫活性。特别是,(取代的)苯磺酰氨基三氮酮I-9 (LC 50 = 2.9869 mg/kg)、I-10 (LC 50 = 3.4957 mg/kg)、I-11 (LC 50= 2.3154 mg/kg)、I-12 (LC 50 = 4.1614 mg/kg)、I-13 (LC 50 = 2.1690 mg/kg) 和I-14 (LC 50 = 3.0077 mg/kg) 表现出更高的杀蚜性活性高于吡蚜酮 (LC 50 = 6.0635 mg/kg)。所有单磺酰基取代的 4-氨基三嗪酮都比相应的二磺酰基取代化合物具有更好的活性。此外,一些衍生物还表现出对淡色库蚊的杀虫活性。间棉铃虫,粘虫,和欧洲玉米螟,化合物I-18和I-21对C. pipiens pallens表现出良好的杀幼虫活性(LC 50值分别为 0.6929 和 0.2592 mg/kg)。































 京公网安备 11010802027423号
京公网安备 11010802027423号