当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phase Transformation Mechanism of Amorphous Calcium Phosphate to Hydroxyapatite Investigated by Liquid-Cell Transmission Electron Microscopy
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2021-08-10 , DOI: 10.1021/acs.cgd.1c00503 Biao Jin 1, 2 , Zhaoming Liu 1 , Changyu Shao 1 , Jiajun Chen 2 , Lili Liu 2 , Ruikang Tang 1 , James J. De Yoreo 2, 3
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2021-08-10 , DOI: 10.1021/acs.cgd.1c00503 Biao Jin 1, 2 , Zhaoming Liu 1 , Changyu Shao 1 , Jiajun Chen 2 , Lili Liu 2 , Ruikang Tang 1 , James J. De Yoreo 2, 3
Affiliation
|
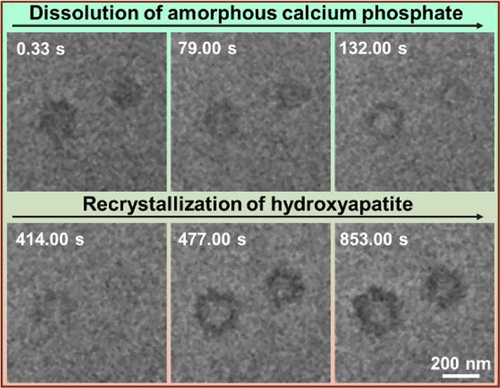
Crystallization via phase transformation of a metastable precursor is a ubiquitous and effective strategy used by living systems to direct the growth of crystalline nanomaterials with remarkable functional properties. However, determining the exact process by which transformation occurs at the nanoscale is a difficult challenge. Here, the recrystallization process of amorphous calcium phosphate (ACP) to hydroxyapatite (HAP) is explored by liquid-cell transmission electron microscopy. The effect of confinement in the liquid-cell is found to increase the size of ACP nanoparticles. In the presence of Mg2+, these large ACP nanoparticles transform to HAP by first dissolving from the interior to create a hollow structure, after which HAP forms preferentially on the surface and then subsequently in the bulk solution. We propose that the preferential dissolution within ACP particles is due to a change in the structure and/or chemistry of the ACP surface, likely associated with dehydration before crystallization of HAP. These results imply an important role of the confined environment of the liquid-cell in regulating the size of ACP particles, which then affects the surface structure and the detailed dissolution–recrystallization pathway. Moreover, we stress the key role of Mg2+ in controlling HAP formation by stabilizing ACP via reduction in ACP solubility. This work provides a better understanding of the roles of additives and confinement during the phase transformation of ACP to HAP through dissolution and recrystallization.
中文翻译:

液体细胞透射电子显微镜研究无定形磷酸钙向羟基磷灰石的相变机理
通过亚稳态前体的相变结晶是一种普遍存在且有效的策略,生命系统用于指导具有显着功能特性的晶体纳米材料的生长。然而,确定在纳米尺度上发生转变的确切过程是一项艰巨的挑战。在这里,无定形磷酸钙 (ACP) 向羟基磷灰石 (HAP) 的重结晶过程通过液细胞透射电子显微镜进行了探索。发现液体细胞中的限制效应会增加 ACP 纳米颗粒的尺寸。在 Mg 2+存在下,这些大的 ACP 纳米粒子通过首先从内部溶解以形成中空结构而转变为 HAP,之后 HAP 优先在表面形成,然后在本体溶液中形成。我们认为 ACP 颗粒内的优先溶解是由于 ACP 表面的结构和/或化学变化,可能与 HAP 结晶之前的脱水有关。这些结果意味着液池的密闭环境在调节 ACP 颗粒的尺寸方面起着重要作用,进而影响表面结构和详细的溶解-再结晶途径。此外,我们强调 Mg 2+的关键作用通过降低 ACP 溶解度来稳定 ACP 来控制 HAP 形成。这项工作提供了对添加剂和限制在 ACP 通过溶解和重结晶相转变为 HAP 过程中的作用的更好理解。
更新日期:2021-09-01
中文翻译:

液体细胞透射电子显微镜研究无定形磷酸钙向羟基磷灰石的相变机理
通过亚稳态前体的相变结晶是一种普遍存在且有效的策略,生命系统用于指导具有显着功能特性的晶体纳米材料的生长。然而,确定在纳米尺度上发生转变的确切过程是一项艰巨的挑战。在这里,无定形磷酸钙 (ACP) 向羟基磷灰石 (HAP) 的重结晶过程通过液细胞透射电子显微镜进行了探索。发现液体细胞中的限制效应会增加 ACP 纳米颗粒的尺寸。在 Mg 2+存在下,这些大的 ACP 纳米粒子通过首先从内部溶解以形成中空结构而转变为 HAP,之后 HAP 优先在表面形成,然后在本体溶液中形成。我们认为 ACP 颗粒内的优先溶解是由于 ACP 表面的结构和/或化学变化,可能与 HAP 结晶之前的脱水有关。这些结果意味着液池的密闭环境在调节 ACP 颗粒的尺寸方面起着重要作用,进而影响表面结构和详细的溶解-再结晶途径。此外,我们强调 Mg 2+的关键作用通过降低 ACP 溶解度来稳定 ACP 来控制 HAP 形成。这项工作提供了对添加剂和限制在 ACP 通过溶解和重结晶相转变为 HAP 过程中的作用的更好理解。

































 京公网安备 11010802027423号
京公网安备 11010802027423号