当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis of Atropisomeric Biaryls by Pd-Catalyzed Asymmetric Buchwald–Hartwig Amination
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-08-09 , DOI: 10.1002/anie.202108747 Peng Zhang 1 , Xiao-Mei Wang 1 , Qi Xu 1 , Chang-Qiu Guo 1 , Peng Wang 1 , Chuan-Jun Lu 1 , Ren-Rong Liu 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-08-09 , DOI: 10.1002/anie.202108747 Peng Zhang 1 , Xiao-Mei Wang 1 , Qi Xu 1 , Chang-Qiu Guo 1 , Peng Wang 1 , Chuan-Jun Lu 1 , Ren-Rong Liu 1
Affiliation
|
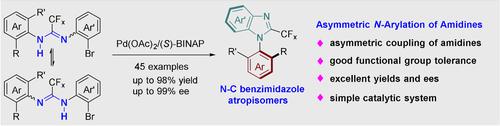
N−C Biaryl atropisomers are prevalent in natural products and bioactive drug molecules. However, the enantioselective synthesis of such molecules has not developed significantly. Particularly, the enantioselective synthesis of N−C biaryl atropisomers by stereoselective metal-catalyzed aryl amination remains unprecedented. Herein, a Pd-catalyzed cross-coupling strategy is presented for the synthesis of N−C axially chiral biaryl molecules. A broad spectrum of N−C axially chiral compounds was obtained with excellent enantioselectivities (up to 99 % ee) and good yields (up to 98 %). The practicality of this reaction was validated in the synthesis of useful biological molecules.
中文翻译:

通过 Pd 催化的不对称 Buchwald-Hartwig 胺化反应对映选择性合成阻转异构联芳基化合物
NC 联芳基阻转异构体普遍存在于天然产物和生物活性药物分子中。然而,此类分子的对映选择性合成尚未显着发展。特别是,通过立体选择性金属催化芳基胺化反应对映选择性合成 NC 联芳基阻转异构体仍然是前所未有的。在此,提出了一种用于合成 NC 轴向手性联芳基分子的 Pd 催化交叉偶联策略。获得了广谱的 NC 轴向手性化合物,具有出色的对映选择性(高达 99 % ee)和良好的产率(高达 98 %)。该反应的实用性在有用的生物分子的合成中得到验证。
更新日期:2021-09-20
中文翻译:

通过 Pd 催化的不对称 Buchwald-Hartwig 胺化反应对映选择性合成阻转异构联芳基化合物
NC 联芳基阻转异构体普遍存在于天然产物和生物活性药物分子中。然而,此类分子的对映选择性合成尚未显着发展。特别是,通过立体选择性金属催化芳基胺化反应对映选择性合成 NC 联芳基阻转异构体仍然是前所未有的。在此,提出了一种用于合成 NC 轴向手性联芳基分子的 Pd 催化交叉偶联策略。获得了广谱的 NC 轴向手性化合物,具有出色的对映选择性(高达 99 % ee)和良好的产率(高达 98 %)。该反应的实用性在有用的生物分子的合成中得到验证。































 京公网安备 11010802027423号
京公网安备 11010802027423号