European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2021-08-10 , DOI: 10.1016/j.ejmech.2021.113764 Shangde Liu 1 , Duo Yuan 1 , Shanshan Li 1 , Roujie Xie 1 , Yi Kong 2 , Xiong Zhu 1
|
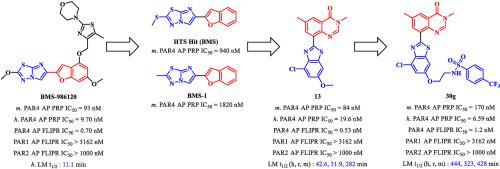
Protease activated receptor 4 (PAR4) is an important target in antiplatelet therapy to reduce the risk of heart attack and thrombotic complications in stroke. PAR4 antagonists can prevent harmful and stable thrombus growth, while retaining initial thrombus formation, by acting on the late diffusion stage of platelet aggregation, and may provide a safer alternative to other antiplatelet agents. To date, only two PAR4 antagonists, BMS-986120 and BMS-986141 have entered clinical trials for thrombosis. Thus, the development of a potent and selective PAR4 antagonist with a novel chemotype is highly desirable. In this study, we explored the activity of quinazolin-4(3H)-one-based PAR4 antagonists, beginning with their IDT analogues. By repeated structural optimisation, we developed a series of highly selective PAR4 antagonists with nanomolar potency on human platelets. Of these, 13 and 30g, with an 8-benzo[d]thiazol-2-yl-substituted quinazolin-4(3H)-one structure, showed optimal activity (h. PAR4-AP PRP IC50 = 19.6 nM and 6.59 nM, respectively) on human platelets. Furthermore, 13 and 30g showed excellent selectivity for PAR4 versus PAR1 and other receptors (IC50s > 10 μM) on human platelets. And 13 and 30g were lack of cross-reactivity for PAR1 or PAR2 (PAR1 AP FLIPR IC50 > 3162 nM, PAR2 AP FLIPR IC50 > 1000 nM) in the calcium mobilization assays. Metabolic stability assays and cytotoxicity tests of 13 and 30g indicated that these compounds could sever as promising drug candidates for the development of novel PAR4 antagonists. In summary, the quinazolin-4(3H)-one-based analogues are the first reported chemotypes with excellent activity and selectivity against PAR4, and, in the current study, we expanded the structural diversity of PAR4 antagonists. The two compounds, 13 and 30g, found in our study could be promising starting points with great potential for further research in antiplatelet therapy.
中文翻译:

基于 quinazolin-4(3H)-one 支架的新型强效蛋白酶激活受体 4 (PAR4) 拮抗剂的合成与评价
蛋白酶激活受体 4 (PAR4) 是抗血小板治疗的重要靶点,可降低中风心脏病发作和血栓并发症的风险。PAR4 拮抗剂通过作用于血小板聚集的晚期扩散阶段,可以防止有害和稳定的血栓生长,同时保留初始血栓形成,并可能为其他抗血小板药物提供更安全的替代品。迄今为止,只有两种 PAR4 拮抗剂BMS-986120和BMS-986141已进入血栓形成的临床试验。因此,非常需要开发具有新化学型的强效和选择性PAR4拮抗剂。在本研究中,我们探讨了 quinazolin-4(3 H)-基于 PAR4 的拮抗剂,从其 IDT 类似物开始。通过反复的结构优化,我们开发了一系列对人血小板具有纳摩尔效力的高选择性 PAR4 拮抗剂。其中,13和30g具有 8-苯并[ d ]噻唑-2-基-取代的 quinazolin-4(3 H )-one 结构,显示出最佳活性(h. PAR4-AP PRP IC 50 = 19.6 nM 和 6.59 nM,分别)在人血小板上。此外,13和30g对人血小板上的 PAR4 与 PAR1 和其他受体(IC 50 s > 10 μM)表现出优异的选择性。还有13和30g在钙动员试验中,PAR1 或 PAR2 缺乏交叉反应性(PAR1 AP FLIPR IC 50 > 3162 nM,PAR2 AP FLIPR IC 50 > 1000 nM)。13克和30 克的代谢稳定性测定和细胞毒性测试表明,这些化合物可以作为开发新型 PAR4 拮抗剂的有希望的候选药物。总之,quinazolin-4(3 H )-one-based 类似物是第一个报道的对 PAR4 具有优异活性和选择性的化学类型,并且在当前的研究中,我们扩展了 PAR4 拮抗剂的结构多样性。两种化合物,13和30g,在我们的研究中发现可能是有希望的起点,具有进一步研究抗血小板治疗的巨大潜力。






























 京公网安备 11010802027423号
京公网安备 11010802027423号