当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Synthesis of Chiral Bicyclo[2.2.1]hepta-2,5-diene Ligands through Rhodium-Catalyzed Asymmetric Arylative Bis-cyclization of a 1,6-Enyne
Organic Letters ( IF 4.9 ) Pub Date : 2021-08-10 , DOI: 10.1021/acs.orglett.1c02088 Chao Sun, He Meng, Chen Chen, Haili Wei, Jialin Ming, Tamio Hayashi
Organic Letters ( IF 4.9 ) Pub Date : 2021-08-10 , DOI: 10.1021/acs.orglett.1c02088 Chao Sun, He Meng, Chen Chen, Haili Wei, Jialin Ming, Tamio Hayashi
|
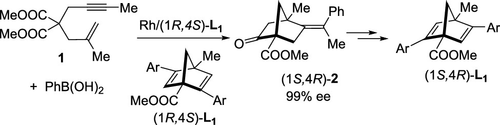
A series of novel chiral diene ligands (1R,4S)-L1, which are based on the bicyclo[2.2.1]heptadiene skeleton and are substituted with methyl and an ester group at the bridgehead carbons, were synthesized through rhodium-catalyzed asymmetric arylative bis-cyclization of 1,6-enyne 1 as a key step. The rhodium catalyst with one of the (1R,4S)-L1 ligands was used for the asymmetric bis-cyclization of 1 giving bicyclic product (1S,4R)-2 of 99% ee, which is a synthetic precursor of (1S,4R)-L1 ligands.
中文翻译:

通过铑催化的1,6-烯炔的不对称芳基双环化不对称合成手性双环[2.2.1]庚-2,5-二烯配体
通过铑合成了一系列基于双环[2.2.1]庚二烯骨架并在桥头碳上被甲基和酯基取代的新型手性二烯配体(1 R, 4 S ) -L 1。作为关键步骤,催化 1,6-烯炔1 的不对称芳基双环化。铑催化剂用的一(1个R, 4小号)-L 1被用于的不对称双-环化的配体1个得到双环产物(1 S, 4 - [R )-2 99%ee值,这是一种合成前体(1 S, 4 R) -L 1配体。
更新日期:2021-08-20
中文翻译:

通过铑催化的1,6-烯炔的不对称芳基双环化不对称合成手性双环[2.2.1]庚-2,5-二烯配体
通过铑合成了一系列基于双环[2.2.1]庚二烯骨架并在桥头碳上被甲基和酯基取代的新型手性二烯配体(1 R, 4 S ) -L 1。作为关键步骤,催化 1,6-烯炔1 的不对称芳基双环化。铑催化剂用的一(1个R, 4小号)-L 1被用于的不对称双-环化的配体1个得到双环产物(1 S, 4 - [R )-2 99%ee值,这是一种合成前体(1 S, 4 R) -L 1配体。































 京公网安备 11010802027423号
京公网安备 11010802027423号