当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Facile and Rapid Room-Temperature Electrosynthesis and Controlled Surface Growth of Fe-MIL-101 and Fe-MIL-101-NH2
ACS Central Science ( IF 12.7 ) Pub Date : 2021-08-10 , DOI: 10.1021/acscentsci.1c00686 Wenbo Wu 1 , Gerald E Decker 1 , Anna E Weaver 1 , Amanda I Arnoff 1 , Eric D Bloch 1 , Joel Rosenthal 1
ACS Central Science ( IF 12.7 ) Pub Date : 2021-08-10 , DOI: 10.1021/acscentsci.1c00686 Wenbo Wu 1 , Gerald E Decker 1 , Anna E Weaver 1 , Amanda I Arnoff 1 , Eric D Bloch 1 , Joel Rosenthal 1
Affiliation
|
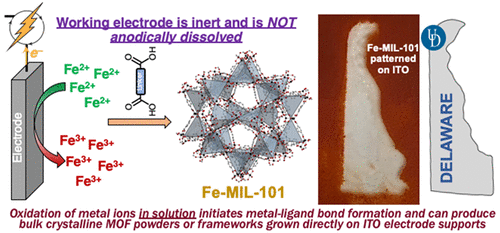
The electrochemical synthesis of metal–organic frameworks (MOFs) has been widely explored but has involved indirect routes, including anodic dissolution of solid metal electrodes or the use of interfacial redox chemistry to generate base equivalents and drive MOF assembly. These methods are limited in scope, as the former relies on the use of an anode consisting of the metal ion to be incorporated into the MOF, and the latter relies on the compatibility of the metal/ligand solution with the probase that is subsequently oxidized or reduced. We report the facile, direct electrochemical syntheses of four iron-based MOFs via controlled potential oxidation of dissolved metal cations. Oxidation of Fe(II) at +0.75 V (vs Ag/Ag+) in a solution containing 2,6-lutidine and terephthalic acid affords highly crystalline Fe-MIL-101. Controlled potential electrolysis with carboxy-functionalized ITO affords Fe-MIL-101 grown directly on the surface of modified electrodes. The methods we report herein represent the first general routes that employ interfacial electrochemistry to alter the oxidation state of metal ions dissolved in solution to directly trigger MOF formation. The reported method is functional group tolerant and will be broadly applicable to the bulk synthesis or surface growth of a range of MOFs based on metal ions with accessible oxidation states.
中文翻译:

Fe-MIL-101 和 Fe-MIL-101-NH2 的简便快速室温电合成和受控表面生长
金属有机框架(MOF)的电化学合成已被广泛探索,但涉及间接途径,包括固体金属电极的阳极溶解或使用界面氧化还原化学来产生碱当量并驱动MOF组装。这些方法的范围有限,因为前者依赖于使用由要掺入 MOF 中的金属离子组成的阳极,而后者依赖于金属/配体溶液与随后被氧化或的探针碱的相容性。减少。我们报告了通过溶解金属阳离子的受控电位氧化来简便、直接地电化学合成四种铁基 MOF。在含有 2,6-二甲基吡啶和对苯二甲酸的溶液中,在 +0.75 V(相对于 Ag/Ag + )下氧化 Fe(II),得到高度结晶的 Fe-MIL-101。使用羧基功能化 ITO 进行控制电位电解,可在修饰电极表面直接生长 Fe-MIL-101。我们在此报告的方法代表了采用界面电化学来改变溶解在溶液中的金属离子的氧化态以直接触发 MOF 形成的第一个一般途径。所报道的方法具有官能团耐受性,将广泛适用于一系列基于具有可接近氧化态的金属离子的 MOF 的本体合成或表面生长。
更新日期:2021-08-25
中文翻译:

Fe-MIL-101 和 Fe-MIL-101-NH2 的简便快速室温电合成和受控表面生长
金属有机框架(MOF)的电化学合成已被广泛探索,但涉及间接途径,包括固体金属电极的阳极溶解或使用界面氧化还原化学来产生碱当量并驱动MOF组装。这些方法的范围有限,因为前者依赖于使用由要掺入 MOF 中的金属离子组成的阳极,而后者依赖于金属/配体溶液与随后被氧化或的探针碱的相容性。减少。我们报告了通过溶解金属阳离子的受控电位氧化来简便、直接地电化学合成四种铁基 MOF。在含有 2,6-二甲基吡啶和对苯二甲酸的溶液中,在 +0.75 V(相对于 Ag/Ag + )下氧化 Fe(II),得到高度结晶的 Fe-MIL-101。使用羧基功能化 ITO 进行控制电位电解,可在修饰电极表面直接生长 Fe-MIL-101。我们在此报告的方法代表了采用界面电化学来改变溶解在溶液中的金属离子的氧化态以直接触发 MOF 形成的第一个一般途径。所报道的方法具有官能团耐受性,将广泛适用于一系列基于具有可接近氧化态的金属离子的 MOF 的本体合成或表面生长。































 京公网安备 11010802027423号
京公网安备 11010802027423号