当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New Findings of Ferrate(VI) Oxidation Mechanism from Its Degradation of Alkene Imidazole Ionic Liquids
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-08-09 , DOI: 10.1021/acs.est.1c03348 Beibei Li 1 , Ruixue Guo 1 , Jie Tian 1 , Zunyao Wang 1 , Ruijuan Qu 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-08-09 , DOI: 10.1021/acs.est.1c03348 Beibei Li 1 , Ruixue Guo 1 , Jie Tian 1 , Zunyao Wang 1 , Ruijuan Qu 1
Affiliation
|
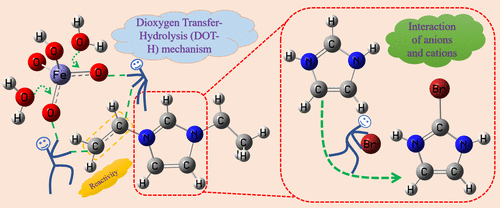
Chemical reactivity, kinetics, degradation pathways and mechanisms, and ecotoxicity of the oxidation of 1-vinyl-3-ethylimidazolium bromide ([VEIm]Br), the most common alternative to organic solvents, by Fe(VI) (HFeO4–) were studied by lab experiments and theoretical calculations. Results show that Fe(VI) can efficiently remove VEIm through the dioxygen transfer-hydrolysis mechanism, which has not been reported yet. The reactivity of VEIm toward Fe(VI) mainly depends on the double bonds in the side chain of VEIm. The second-order rate constant for VEIm was 629.45 M–1 s–1 at pH 7.0 and 25 °C. Typical water constituents, except for SO32–, Cl–, and Cu2+, had no obvious effects on the oxidation. The oxidation products were determined by high-performance liquid chromatography hybrid quadrupole time-of-flight mass spectrometry, which proves that there were interactions between the oxidation intermediates of the anion and cation parts of [VEIm]Br during the degradation process. The structures of related products and oxidation mechanisms were further rationalized by theoretical calculations. The ecotoxicity of products from the three oxidation pathways all showed a trend of increase after the initial decrease. We hope that the findings of this work can give researchers some new inspirations on Fe(VI) degradation of other alkene-containing contaminants.
中文翻译:

高铁酸盐(VI)氧化机理的新发现从烯烃咪唑离子液体的降解
Fe(VI) (HFeO 4 – )氧化 1-乙烯基-3-乙基咪唑鎓溴化物 ([VEIm]Br)(有机溶剂的最常见替代品)的化学反应性、动力学、降解途径和机制以及生态毒性是通过实验室实验和理论计算研究。结果表明,Fe(VI)可以通过分子氧转移-水解机制有效去除VEIm,但尚未见报道。VEIm 对 Fe(VI) 的反应性主要取决于 VEIm 侧链中的双键。VEIm 的二级速率常数在 pH 7.0 和 25 °C 时为 629.45 M –1 s –1。典型的水成分,SO 3 2–、Cl –和 Cu 2+除外, 对氧化无明显影响。通过高效液相色谱混合四极杆飞行时间质谱法测定氧化产物,证明在降解过程中[VEIm]Br的阴离子和阳离子部分的氧化中间体之间存在相互作用。通过理论计算进一步合理化了相关产物的结构和氧化机制。三种氧化途径产物的生态毒性均呈先下降后上升的趋势。我们希望这项工作的发现可以为研究人员对其他含烯烃污染物的 Fe(VI) 降解提供一些新的启发。
更新日期:2021-09-07
中文翻译:

高铁酸盐(VI)氧化机理的新发现从烯烃咪唑离子液体的降解
Fe(VI) (HFeO 4 – )氧化 1-乙烯基-3-乙基咪唑鎓溴化物 ([VEIm]Br)(有机溶剂的最常见替代品)的化学反应性、动力学、降解途径和机制以及生态毒性是通过实验室实验和理论计算研究。结果表明,Fe(VI)可以通过分子氧转移-水解机制有效去除VEIm,但尚未见报道。VEIm 对 Fe(VI) 的反应性主要取决于 VEIm 侧链中的双键。VEIm 的二级速率常数在 pH 7.0 和 25 °C 时为 629.45 M –1 s –1。典型的水成分,SO 3 2–、Cl –和 Cu 2+除外, 对氧化无明显影响。通过高效液相色谱混合四极杆飞行时间质谱法测定氧化产物,证明在降解过程中[VEIm]Br的阴离子和阳离子部分的氧化中间体之间存在相互作用。通过理论计算进一步合理化了相关产物的结构和氧化机制。三种氧化途径产物的生态毒性均呈先下降后上升的趋势。我们希望这项工作的发现可以为研究人员对其他含烯烃污染物的 Fe(VI) 降解提供一些新的启发。






























 京公网安备 11010802027423号
京公网安备 11010802027423号