Molecular Therapy - Methods & Clinical Development ( IF 4.6 ) Pub Date : 2021-08-08 , DOI: 10.1016/j.omtm.2021.07.007 Shi Cheng 1 , Marcel M van Gaalen 2 , Mathias Bähr 1 , Enrique Garea-Rodriguez 2 , Sebastian Kügler 1
|
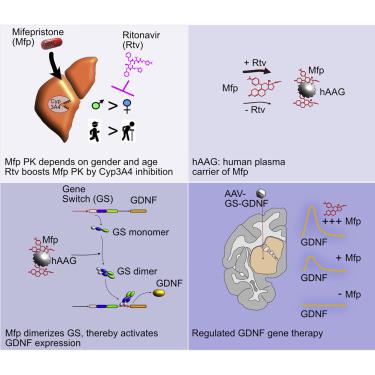
Gene therapy in its current design is an irreversible process. It cannot be stopped in case of unwanted side effects, nor can expression levels of therapeutics be adjusted to individual patient’s needs. Thus, the Gene-Switch (GS) system for pharmacologically regulable neurotrophic factor expression was established for treatment of parkinsonian patients. Mifepristone, the synthetic steroid used to control transgene expression of the GS vector, is an approved clinical drug. However, pharmacokinetics and -dynamics of mifepristone vary considerably between different experimental animal species and depend on age and gender. In humans, but not in any other species, mifepristone binds to a high-affinity plasma carrier protein. We now demonstrate that the formulation of mifepristone can have robust impact on its ability to activate the GS system. Furthermore, we show that a pharmacological booster, ritonavir (Rtv), robustly enhances the pharmacological effect of mifepristone, and allows it to overcome gender- and species-specific pharmacokinetic and -dynamic issues. Most importantly, we demonstrate that the GS vector can be efficiently controlled by mifepristone in the presence of its human plasma carrier protein, α1-acid glycoprotein, in a “humanized” rat model. Thus, we have substantially improved the applicability of the GS vector toward therapeutic use in patients.
中文翻译:

对 AAV-Gene-Switch 载体进行优化的药理学控制,用于可调控的基因治疗
目前设计的基因治疗是一个不可逆转的过程。在出现不需要的副作用的情况下不能停止,也不能根据个体患者的需要调整治疗剂的表达水平。因此,建立了用于药理学上可调节的神经营养因子表达的基因开关(GS)系统,用于治疗帕金森病患者。米非司酮是一种用于控制 GS 载体转基因表达的合成类固醇,是一种获批的临床药物。然而,米非司酮的药代动力学和动力学在不同的实验动物物种之间差异很大,并且取决于年龄和性别。在人类中,但在任何其他物种中,米非司酮与高亲和力血浆载体蛋白结合。我们现在证明米非司酮的配方可以对其激活 GS 系统的能力产生强大的影响。此外,我们表明,药理学助推器利托那韦 (Rtv) 可以有力地增强米非司酮的药理作用,并使其能够克服性别和物种特异性的药代动力学和动力学问题。最重要的是,我们证明在“人源化”大鼠模型中,米非司酮可以在其人血浆载体蛋白α1-酸性糖蛋白存在的情况下有效控制 GS 载体。因此,我们大大提高了 GS 载体在患者治疗中的适用性。我们证明,在“人源化”大鼠模型中,米非司酮可以在其人血浆载体蛋白α1-酸性糖蛋白存在的情况下有效控制 GS 载体。因此,我们大大提高了 GS 载体在患者治疗中的适用性。我们证明,在“人源化”大鼠模型中,米非司酮可以在其人血浆载体蛋白α1-酸性糖蛋白存在的情况下有效控制 GS 载体。因此,我们大大提高了 GS 载体在患者治疗中的适用性。































 京公网安备 11010802027423号
京公网安备 11010802027423号