Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2021-08-08 , DOI: 10.1016/j.bmc.2021.116346 Huarong Yang 1 , Qing Li 2 , Mingzhi Su 2 , Fang Luo 2 , Yahua Liu 3 , Daoping Wang 2 , Yanhua Fan 2
|
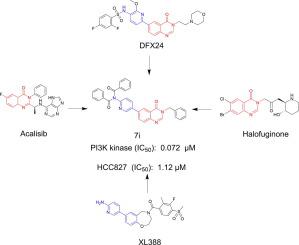
Abnormal activation of the PI3K/Akt pathway is demonstrated in most of human malignant tumors via regulation of proliferation, cell cycle, and apoptosis. Therefore, drug discovery and development of targeting the PI3K/Akt pathway has attracted great interest of researchers in the development of anticancer drugs. In this study, fifteen 6-(pyridin-3-yl) quinazolin-4(3H)-one derivatives were designed and synthesized. Anticancer activities of the synthetic compounds were evaluated and the potential mechanisms were explored. Several compounds showed certain proliferation inhibitory activity against the tested cancer cells including human non-small cell lung cancer (NSCLC) HCC827, human neuroblastoma SH-SY5Y and hepatocellular carcinoma LM3 cells. Among them, compound 7i and 7m showed the best inhibitory activity against all the cancer cell lines and more active against HCC827 cells with IC50 values of 1.12 μM and 1.20 μM, respectively. In addition, 7i and 7m showed lower inhibitory activity against H7702 cells (human normal liver cells) with IC50 values of 8.66 μM and 10.89 μM, respectively, nearly 8-fold lower than that in HCC827 cells. These results suggested that compounds 7i and 7m had certain selectivity to tumor cells, compared to human normal cells. Further biological studies indicated 7i induced G2/M phase arrests and cell apoptosis of HCC827 cells via PI3K/Akt and caspase dependent pathway. Together, these novel 6-(pyridin-3-yl) quinazolin-4(3H)-one derivatives such as compound 7i and 7m might be lead compounds for development of potential anti-cancer drugs.
中文翻译:

通过 PI3K 抑制作为潜在抗癌剂的新型 6-(pyridin-3-yl) quinazolin-4(3H)-one 衍生物的设计、合成和生物学评价
PI3K/Akt 通路的异常激活通过调节增殖、细胞周期和细胞凋亡在大多数人类恶性肿瘤中得到证实。因此,靶向PI3K/Akt通路的药物发现和开发引起了研究人员对抗癌药物开发的极大兴趣。在这项研究中,设计并合成了15 种 6-(pyridin-3-yl) quinazolin-4(3 H )-one 衍生物。评估了合成化合物的抗癌活性,并探索了潜在的机制。几种化合物对测试的癌细胞显示出一定的增殖抑制活性,包括人非小细胞肺癌 (NSCLC) HCC827、人神经母细胞瘤 SH-SY5Y 和肝细胞癌 LM3 细胞。其中,复合7i和7m对所有癌细胞系显示出最佳抑制活性,对 HCC827 细胞显示出更高的活性,IC 50值分别为 1.12 μM 和 1.20 μM。此外,7i和7m对 H7702 细胞(人正常肝细胞)的抑制活性较低,IC 50值分别为 8.66 μM 和 10.89 μM,比 HCC827 细胞低近 8 倍。这些结果表明,与人类正常细胞相比,化合物7i和7m对肿瘤细胞具有一定的选择性。进一步的生物学研究表明7i通过 PI3K/Akt 和半胱天冬酶依赖性途径诱导 HCC827 细胞的 G2/M 期停滞和细胞凋亡。总之,这些新的 6-(pyridin-3-yl) quinazolin-4(3 H )-one 衍生物如化合物7i和7m可能是开发潜在抗癌药物的先导化合物。






























 京公网安备 11010802027423号
京公网安备 11010802027423号