当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Closed-Loop System Driven by ADP Phosphorylation from Pyrophosphate Affords Equimolar Transformation of ATP to 3′-Phosphoadenosine-5′-phosphosulfate
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-08-06 , DOI: 10.1021/acscatal.1c02004 Ruirui Xu 1, 2, 3 , Yang Wang 1, 2, 3 , Hao Huang 1, 2, 3 , Xuerong Jin 1, 2, 3 , Jianghua Li 1, 2, 3 , Guocheng Du 1, 2, 3 , Zhen Kang 1, 2, 3
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-08-06 , DOI: 10.1021/acscatal.1c02004 Ruirui Xu 1, 2, 3 , Yang Wang 1, 2, 3 , Hao Huang 1, 2, 3 , Xuerong Jin 1, 2, 3 , Jianghua Li 1, 2, 3 , Guocheng Du 1, 2, 3 , Zhen Kang 1, 2, 3
Affiliation
|
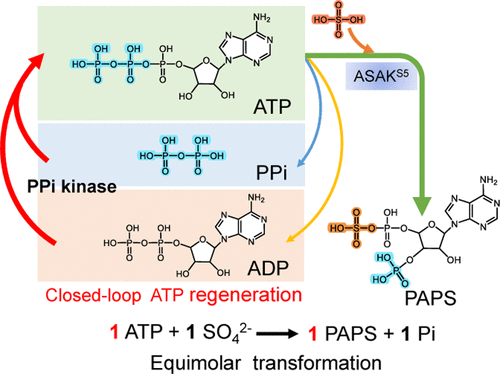
3′-Phosphoadenosine-5′-phosphosulfate (PAPS) is a universal sulfate group donor for all biological sulfation reactions in living organisms. Ambitions to biomanufacture sulfate-containing compounds such as heparin and chondroitin sulfate also promote the study on PAPS in vitro synthesis. However, the established enzymatic synthesis of PAPS faces hurdles of the natural low theoretical transformation rate of 50% (two ATP to one PAPS) and high cost. Here, we developed a PAPS synthesis route with 100% theoretical transformation rate which affords equimolar transformation of ATP to PAPS. By fusing the identified adenosine 5′-triphosphate sulfurylase and adenosine 5′-phosphosulfate kinase from different species, we created an artificial active bifunctional enzyme to directly convert ATP to PAPS. To maximize the conversion from ATP to PAPS, a polyphosphate (polyP)-dependent ATP regeneration system was designed and engineered by screening polyP kinases which consumes the low-cost polyP as the phosphate donor. In addition, we found that PPi could be used as the phosphate donor for phosphorylating ADP to ATP by polyP kinases. After demonstration of the wide distribution of PPi kinase activity in polyP kinases, a closed-loop ATP regeneration route was thereupon created to convert one ATP to one PAPS in theory. Using PPi as the phosphate donor, the conversion rate of ATP to PAPS reached 92.3%. The efficient enzymatic route that is constructed here for PAPS synthesis with low cost would boost the biosynthesis of sulfated compounds and peptides.
中文翻译:

由焦磷酸盐的 ADP 磷酸化驱动的闭环系统提供 ATP 到 3'-磷酸腺苷-5'-磷酸硫酸盐的等摩尔转化
3'-Phosphoadenosine-5'-phosphosulfate (PAPS) 是生物体内所有生物硫酸化反应的通用硫酸基供体。对生物制造含硫酸盐化合物如肝素和硫酸软骨素的雄心也促进了体外PAPS的研究合成。然而,已建立的 PAPS 酶法合成面临着 50% 的天然低理论转化率(两个 ATP 到一个 PAPS)和高成本的障碍。在这里,我们开发了一种具有 100% 理论转化率的 PAPS 合成路线,可将 ATP 等摩尔转化为 PAPS。通过融合来自不同物种的已鉴定的腺苷 5'-三磷酸硫酸化酶和腺苷 5'-磷酸硫酸激酶,我们创造了一种人工活性双功能酶,可将 ATP 直接转化为 PAPS。为了最大限度地提高从 ATP 到 PAPS 的转化率,通过筛选消耗低成本 polyP 作为磷酸盐供体的 polyP 激酶,设计和设计了一种多磷酸盐 (polyP) 依赖性 ATP 再生系统。此外,我们发现 PPi 可用作磷酸盐供体,用于通过 polyP 激酶将 ADP 磷酸化为 ATP。在证明 PPi 激酶活性在 polyP 激酶中的广泛分布之后,于是创建了一条闭环 ATP 再生路线,理论上将一种 ATP 转化为一种 PAPS。使用PPi作为磷酸盐供体,ATP向PAPS的转化率达到92.3%。这里构建的用于 PAPS 合成的低成本高效酶促路线将促进硫酸化化合物和肽的生物合成。
更新日期:2021-08-20
中文翻译:

由焦磷酸盐的 ADP 磷酸化驱动的闭环系统提供 ATP 到 3'-磷酸腺苷-5'-磷酸硫酸盐的等摩尔转化
3'-Phosphoadenosine-5'-phosphosulfate (PAPS) 是生物体内所有生物硫酸化反应的通用硫酸基供体。对生物制造含硫酸盐化合物如肝素和硫酸软骨素的雄心也促进了体外PAPS的研究合成。然而,已建立的 PAPS 酶法合成面临着 50% 的天然低理论转化率(两个 ATP 到一个 PAPS)和高成本的障碍。在这里,我们开发了一种具有 100% 理论转化率的 PAPS 合成路线,可将 ATP 等摩尔转化为 PAPS。通过融合来自不同物种的已鉴定的腺苷 5'-三磷酸硫酸化酶和腺苷 5'-磷酸硫酸激酶,我们创造了一种人工活性双功能酶,可将 ATP 直接转化为 PAPS。为了最大限度地提高从 ATP 到 PAPS 的转化率,通过筛选消耗低成本 polyP 作为磷酸盐供体的 polyP 激酶,设计和设计了一种多磷酸盐 (polyP) 依赖性 ATP 再生系统。此外,我们发现 PPi 可用作磷酸盐供体,用于通过 polyP 激酶将 ADP 磷酸化为 ATP。在证明 PPi 激酶活性在 polyP 激酶中的广泛分布之后,于是创建了一条闭环 ATP 再生路线,理论上将一种 ATP 转化为一种 PAPS。使用PPi作为磷酸盐供体,ATP向PAPS的转化率达到92.3%。这里构建的用于 PAPS 合成的低成本高效酶促路线将促进硫酸化化合物和肽的生物合成。































 京公网安备 11010802027423号
京公网安备 11010802027423号