当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction intermediate-mediated electrocatalyst synthesis favors specified facet and defect exposure for efficient nitrate–ammonia conversion
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2021-07-16 , DOI: 10.1039/d1ee01731d Qi Hu 1, 2, 3, 4 , Yongjie Qin 1, 2, 3, 4 , Xiaodeng Wang 1, 2, 3, 4 , Ziyu Wang 1, 2, 3, 4 , Xiaowan Huang 1, 2, 3, 4 , Hongju Zheng 1, 2, 3, 4 , Keru Gao 1, 2, 3, 4 , Hengpan Yang 1, 2, 3, 4 , Peixin Zhang 1, 2, 3, 4 , Minhua Shao 5, 6, 7, 8 , Chuanxin He 1, 2, 3, 4
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2021-07-16 , DOI: 10.1039/d1ee01731d Qi Hu 1, 2, 3, 4 , Yongjie Qin 1, 2, 3, 4 , Xiaodeng Wang 1, 2, 3, 4 , Ziyu Wang 1, 2, 3, 4 , Xiaowan Huang 1, 2, 3, 4 , Hongju Zheng 1, 2, 3, 4 , Keru Gao 1, 2, 3, 4 , Hengpan Yang 1, 2, 3, 4 , Peixin Zhang 1, 2, 3, 4 , Minhua Shao 5, 6, 7, 8 , Chuanxin He 1, 2, 3, 4
Affiliation
|
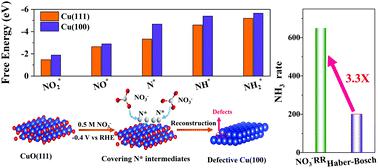
The electrochemical nitrate (NO3−) reduction reaction (NO3−RR), with much faster kinetics than the nitrogen (N2) reduction, provides new opportunities to harvest ammonia (NH3) under ambient conditions. However, the NH3 production rate of NO3−RR is still much inferior to that of the industrial Haber–Bosch route due to the lack of robust electrocatalysts for suppressing the hydrogen evolution reaction (HER) at large current densities. Herein, we demonstrate an electrocatalyst synthesis strategy based on the in situ electrochemical reduction of ultrathin copper-oxide nanobelts under NO3−RR conditions, which favorably exposes Cu(100) facets and abundant surface defects, thereby markedly facilitating the NO3−RR yet hindering the HER. We discover that the intermediates of NO3−RR (i.e., N*) can serve as capping agents for controlling the exposed facets during the reduction. Impressively, in alkaline media, the NO3−RR catalyzed by defective Cu(100) facets gives a NH3 yield rate which is 2.3-fold higher than that of the Haber–Bosch process. The synergy of Cu(100) facets and defects, which upshifts the d band center of Cu, is the key to excellent performance. The reaction intermediate-mediated strategy demonstrated in this study offers a fresh concept and robust methodology for directional electrocatalyst synthesis to achieve markedly enhanced performance.
中文翻译:

反应中间体介导的电催化剂合成有利于高效硝酸盐-氨转化的特定方面和缺陷暴露
电化学硝酸盐 (NO 3 - ) 还原反应 (NO 3 - RR) 的动力学比氮 (N 2 ) 还原快得多,为在环境条件下收集氨 (NH 3 )提供了新的机会。然而,由于缺乏强大的电催化剂来抑制大电流密度下的析氢反应(HER),NO 3 - RR的 NH 3产率仍远低于工业 Haber-Bosch 路线。在此,我们展示了一种基于超薄氧化铜纳米带在 NO 3 -下原位电化学还原的电催化剂合成策略。RR条件,有利地暴露Cu(100)面和丰富的表面缺陷,从而显着促进NO 3 - RR但阻碍了HER。我们发现NO 3 - RR的中间体(即N*)可以作为在还原过程中控制暴露面的封端剂。令人印象深刻的是,在碱性介质中,由有缺陷的 Cu(100) 小平面催化的 NO 3 − RR 产生了 NH 3产率比 Haber-Bosch 工艺高 2.3 倍。Cu(100) 晶面和缺陷的协同作用使 Cu 的 d 带中心上移,是获得优异性能的关键。本研究中展示的反应中间体介导的策略为定向电催化剂合成提供了一个全新的概念和强大的方法,以实现显着增强的性能。
更新日期:2021-08-07
中文翻译:

反应中间体介导的电催化剂合成有利于高效硝酸盐-氨转化的特定方面和缺陷暴露
电化学硝酸盐 (NO 3 - ) 还原反应 (NO 3 - RR) 的动力学比氮 (N 2 ) 还原快得多,为在环境条件下收集氨 (NH 3 )提供了新的机会。然而,由于缺乏强大的电催化剂来抑制大电流密度下的析氢反应(HER),NO 3 - RR的 NH 3产率仍远低于工业 Haber-Bosch 路线。在此,我们展示了一种基于超薄氧化铜纳米带在 NO 3 -下原位电化学还原的电催化剂合成策略。RR条件,有利地暴露Cu(100)面和丰富的表面缺陷,从而显着促进NO 3 - RR但阻碍了HER。我们发现NO 3 - RR的中间体(即N*)可以作为在还原过程中控制暴露面的封端剂。令人印象深刻的是,在碱性介质中,由有缺陷的 Cu(100) 小平面催化的 NO 3 − RR 产生了 NH 3产率比 Haber-Bosch 工艺高 2.3 倍。Cu(100) 晶面和缺陷的协同作用使 Cu 的 d 带中心上移,是获得优异性能的关键。本研究中展示的反应中间体介导的策略为定向电催化剂合成提供了一个全新的概念和强大的方法,以实现显着增强的性能。































 京公网安备 11010802027423号
京公网安备 11010802027423号