当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cobalt-Catalyzed Diastereo- and Enantioselective Reductive Allyl Additions to Aldehydes with Allylic Alcohol Derivatives via Allyl Radical Intermediates
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-08-05 , DOI: 10.1021/jacs.1c05690
Lei Wang 1 , Lifan Wang 1 , Mingxia Li 1 , Qinglei Chong 1 , Fanke Meng 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-08-05 , DOI: 10.1021/jacs.1c05690
Lei Wang 1 , Lifan Wang 1 , Mingxia Li 1 , Qinglei Chong 1 , Fanke Meng 1
Affiliation
|
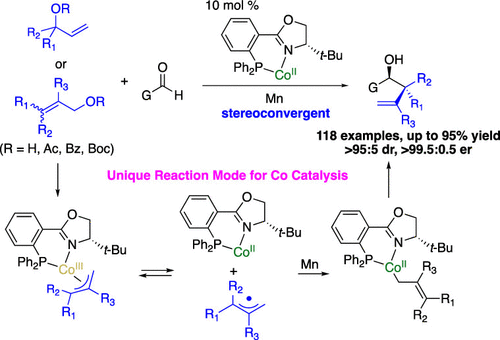
Catalytic generation of ambiphilic π-allyl–metal complexes and their utility in enantioselective transformations constitutes a powerful approach for introduction of allyl groups to a molecule. Herein an unprecedented cobalt-catalyzed highly site-, diastereo-, and enantioselective protocol for stereoselective formation of nucleophilic allyl–Co(II) complexes followed by addition to aldehydes is presented. The reaction features diastereo- and enantioconvergent conversion of easily accessible allylic alcohol derivatives to diversified enantioenriched homoallylic alcohols with a remarkably broad scope of allyl groups that can be introduced. Mechanistic studies indicated that allyl radical intermediates were involved in this process. These new discoveries establish a new strategy for development of enantioselective transformations through capture of radicals by chiral Co complexes, pushing forward the frontier of Co complexes for enantioselective catalysis.
中文翻译:

钴催化的非对映选择性和对映选择性还原烯丙基通过烯丙基中间体与烯丙醇衍生物加成到醛
催化生成两亲性 π-烯丙基-金属配合物及其在对映选择性转化中的应用构成了将烯丙基引入分子的有效方法。本文提出了一种前所未有的钴催化的高度位点、非对映和对映选择性方案,用于立体选择性形成亲核烯丙基-Co(II) 配合物,然后加入醛。该反应的特点是将容易获得的烯丙醇衍生物非对映和对映收敛转化为多样化的富含对映体的高烯丙醇,其中可以引入的烯丙基范围非常广泛。机理研究表明,烯丙基自由基中间体参与了这一过程。
更新日期:2021-08-19
中文翻译:

钴催化的非对映选择性和对映选择性还原烯丙基通过烯丙基中间体与烯丙醇衍生物加成到醛
催化生成两亲性 π-烯丙基-金属配合物及其在对映选择性转化中的应用构成了将烯丙基引入分子的有效方法。本文提出了一种前所未有的钴催化的高度位点、非对映和对映选择性方案,用于立体选择性形成亲核烯丙基-Co(II) 配合物,然后加入醛。该反应的特点是将容易获得的烯丙醇衍生物非对映和对映收敛转化为多样化的富含对映体的高烯丙醇,其中可以引入的烯丙基范围非常广泛。机理研究表明,烯丙基自由基中间体参与了这一过程。































 京公网安备 11010802027423号
京公网安备 11010802027423号