当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interfacial Water Structure as a Descriptor for Its Electro-Reduction on Ni(OH)2-Modified Cu(111)
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-08-04 , DOI: 10.1021/acscatal.1c02673 Andrea Auer 1 , Francisco J Sarabia 2 , Daniel Winkler 1 , Christoph Griesser 1 , Víctor Climent 2 , Juan M Feliu 2 , Julia Kunze-Liebhäuser 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-08-04 , DOI: 10.1021/acscatal.1c02673 Andrea Auer 1 , Francisco J Sarabia 2 , Daniel Winkler 1 , Christoph Griesser 1 , Víctor Climent 2 , Juan M Feliu 2 , Julia Kunze-Liebhäuser 1
Affiliation
|
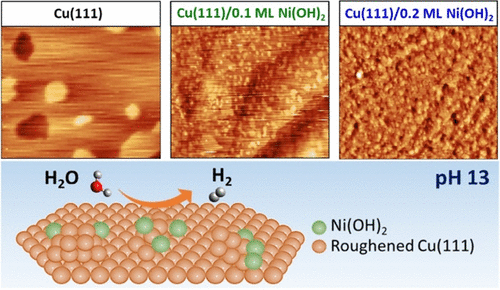
The hydrogen evolution reaction (HER) has been crucial for the development of fundamental knowledge on electrocatalysis and electrochemistry, in general. In alkaline media, many key questions concerning pH-dependent structure–activity relations and the underlying activity descriptors remain unclear. While the presence of Ni(OH)2 deposited on Pt(111) has been shown to highly improve the rate of the HER through the electrode’s bifunctionality, no studies exist on how low coverages of Ni(OH)2 influence the electrocatalytic behavior of Cu surfaces, which is a low-cost alternative to Pt. Here, we demonstrate that Cu(111) modified with 0.1 and 0.2 monolayers (ML) of Ni(OH)2 exhibits an unusual non-linear activity trend with increasing coverage. By combining in situ structural investigations with studies on the interfacial water orientation using electrochemical scanning tunneling microscopy and laser-induced temperature jump experiments, we find a correlation between a particular threshold of surface roughness and the decrease in the ordering of the water network at the interface. The highly disordered water ad-layer close to the onset of the HER, which is only present for 0.2 ML of Ni(OH)2, facilitates the reorganization of the interfacial water molecules to accommodate for charge transfer, thus enhancing the rate of the reaction. These findings strongly suggest a general validity of the interfacial water reorganization as an activity descriptor for the HER in alkaline media.
中文翻译:

界面水结构作为其对 Ni(OH)2 改性 Cu(111) 电还原的描述
一般来说,析氢反应(HER)对于电催化和电化学基础知识的发展至关重要。在碱性介质中,许多关于 pH 依赖性结构-活性关系和潜在活性描述符的关键问题仍不清楚。虽然沉积在 Pt(111) 上的 Ni(OH) 2的存在已被证明通过电极的双功能性大大提高了 HER 的速率,但没有关于 Ni(OH) 2 的低覆盖率如何影响 Cu 的电催化行为的研究表面,这是 Pt 的低成本替代品。在这里,我们证明了用 0.1 和 0.2 个单层 (ML) Ni(OH) 2改性的 Cu(111)表现出不寻常的非线性活动趋势,随着覆盖率的增加。通过组合通过使用电化学扫描隧道显微镜和激光诱导温度跳跃实验研究界面水取向的原位结构研究,我们发现表面粗糙度的特定阈值与界面处水网络有序性的降低之间存在相关性。接近 HER 开始的高度无序的水广告层,仅存在于 0.2 毫升的 Ni(OH) 2,促进界面水分子的重组以适应电荷转移,从而提高反应速率. 这些发现强烈表明界面水重组作为碱性介质中 HER 活性描述符的普遍有效性。
更新日期:2021-08-20
中文翻译:

界面水结构作为其对 Ni(OH)2 改性 Cu(111) 电还原的描述
一般来说,析氢反应(HER)对于电催化和电化学基础知识的发展至关重要。在碱性介质中,许多关于 pH 依赖性结构-活性关系和潜在活性描述符的关键问题仍不清楚。虽然沉积在 Pt(111) 上的 Ni(OH) 2的存在已被证明通过电极的双功能性大大提高了 HER 的速率,但没有关于 Ni(OH) 2 的低覆盖率如何影响 Cu 的电催化行为的研究表面,这是 Pt 的低成本替代品。在这里,我们证明了用 0.1 和 0.2 个单层 (ML) Ni(OH) 2改性的 Cu(111)表现出不寻常的非线性活动趋势,随着覆盖率的增加。通过组合通过使用电化学扫描隧道显微镜和激光诱导温度跳跃实验研究界面水取向的原位结构研究,我们发现表面粗糙度的特定阈值与界面处水网络有序性的降低之间存在相关性。接近 HER 开始的高度无序的水广告层,仅存在于 0.2 毫升的 Ni(OH) 2,促进界面水分子的重组以适应电荷转移,从而提高反应速率. 这些发现强烈表明界面水重组作为碱性介质中 HER 活性描述符的普遍有效性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号