当前位置:
X-MOL 学术
›
J. Cell. Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure-based virtual screening and computational study towards identification of novel inhibitors of hypoxanthine-guanine phosphoribosyltransferase of Trypanosoma cruzi
Journal of Cellular Biochemistry ( IF 3.0 ) Pub Date : 2021-08-04 , DOI: 10.1002/jcb.30122
Venkataramani Malathi Vidhya 1 , Karthe Ponnuraj 1
Journal of Cellular Biochemistry ( IF 3.0 ) Pub Date : 2021-08-04 , DOI: 10.1002/jcb.30122
Venkataramani Malathi Vidhya 1 , Karthe Ponnuraj 1
Affiliation
|
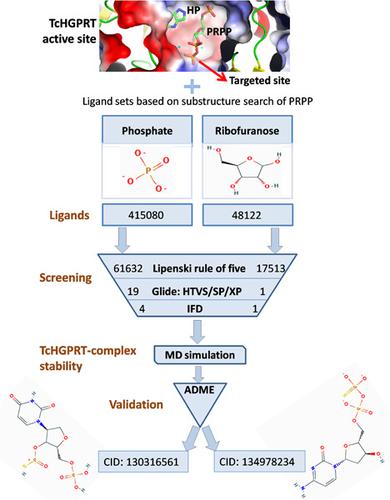
Hypoxanthine-guanine phosphoribosyltransferase (HGPRT) is the key regulatory enzyme of the purine salvage pathway present in the members of trypanosomatids. The parasite solely depends on this pathway for the synthesis of nucleotides due to the absence of the de novo pathway. This study intends to identify putative inhibitors towards Trypanosoma cruzi HGPRT (TcHGPRT). Initial virtual screening was performed with substructures of phosphoribosyl pyrophosphate (PRPP), an original substrate of HGPRT. Twenty compounds that had greater binding energy than the substrate was treated as hits and was further screened and narrowed down through induced fit docking which resulted in top five compounds which was distinguished into two groups based on the ligand occupancy within the PRPP binding site of TcHGPRT. Group-I compounds (PubChem CID 130316561 and 134978234) are analogous to PRPP structure with greater occupancy, were preferred over Group-II compounds which had lesser occupancy than the substrate. However, one compound (22404820) among Group II was chosen for further analysis considering its significant electrostatic interactions. Molecular docking studies revealed the requirement of an electronegative moiety like phosphate group to be present in the ligand due to the presence of metal ions in the substrate binding site. The three chosen compounds along with PRPP were subjected to molecular dynamics analysis, which indicated a strong presence of electrostatic interaction. Considering the dynamic stability of interactions as well as pharmacological properties of ligands based on absorption, distribution, metabolism, excretion prediction, Group-I compounds were selected as lead compounds and were subjected to molecular electrostatic potential analysis to determine the charge distribution of the compound. The overall analysis thus suggests both 130316561 and 134978234 can be used as TcHGPRT inhibitors. Furthermore, these computational results emphasize the requirement of phosphorylated ligands which are essential in mediating electrostatic interactions and to compete with the binding affinity of the original substrate.
中文翻译:

基于结构的虚拟筛选和计算研究鉴定新的克氏锥虫次黄嘌呤-鸟嘌呤磷酸核糖基转移酶抑制剂
次黄嘌呤-鸟嘌呤磷酸核糖基转移酶 (HGPRT) 是锥虫类成员中嘌呤补救途径的关键调节酶。由于缺乏从头途径,寄生虫仅依赖于该途径合成核苷酸。本研究旨在确定对克氏锥虫的推定抑制剂HGPRT (TcHGPRT)。使用 HGPRT 的原始底物磷酸核糖焦磷酸 (PRPP) 的亚结构进行初始虚拟筛选。结合能大于底物的 20 种化合物被视为命中,并通过诱导拟合对接进一步筛选和缩小范围,从而产生前 5 种化合物,根据 TcHGPRT 的 PRPP 结合位点内的配体占有率将其分为两组。第 I 组化合物 (PubChem CID 130316561 和 134978234) 类似于 PRPP 结构,具有更大的占有率,优于占有率低于基材的第 II 组化合物。然而,考虑到其显着的静电相互作用,选择第 II 组中的一种化合物 (22404820) 进行进一步分析。分子对接研究表明,由于底物结合位点中存在金属离子,因此需要在配体中存在像磷酸基团这样的电负性部分。三种选择的化合物与 PRPP 一起进行了分子动力学分析,表明存在强烈的静电相互作用。考虑到相互作用的动态稳定性以及配体基于吸收、分布、代谢、排泄预测的药理特性,选择I组化合物作为先导化合物,并对其进行分子静电势分析以确定化合物的电荷分布。因此,总体分析表明 130316561 和 134978234 均可用作 TcHGPRT 抑制剂。此外,
更新日期:2021-08-04
中文翻译:

基于结构的虚拟筛选和计算研究鉴定新的克氏锥虫次黄嘌呤-鸟嘌呤磷酸核糖基转移酶抑制剂
次黄嘌呤-鸟嘌呤磷酸核糖基转移酶 (HGPRT) 是锥虫类成员中嘌呤补救途径的关键调节酶。由于缺乏从头途径,寄生虫仅依赖于该途径合成核苷酸。本研究旨在确定对克氏锥虫的推定抑制剂HGPRT (TcHGPRT)。使用 HGPRT 的原始底物磷酸核糖焦磷酸 (PRPP) 的亚结构进行初始虚拟筛选。结合能大于底物的 20 种化合物被视为命中,并通过诱导拟合对接进一步筛选和缩小范围,从而产生前 5 种化合物,根据 TcHGPRT 的 PRPP 结合位点内的配体占有率将其分为两组。第 I 组化合物 (PubChem CID 130316561 和 134978234) 类似于 PRPP 结构,具有更大的占有率,优于占有率低于基材的第 II 组化合物。然而,考虑到其显着的静电相互作用,选择第 II 组中的一种化合物 (22404820) 进行进一步分析。分子对接研究表明,由于底物结合位点中存在金属离子,因此需要在配体中存在像磷酸基团这样的电负性部分。三种选择的化合物与 PRPP 一起进行了分子动力学分析,表明存在强烈的静电相互作用。考虑到相互作用的动态稳定性以及配体基于吸收、分布、代谢、排泄预测的药理特性,选择I组化合物作为先导化合物,并对其进行分子静电势分析以确定化合物的电荷分布。因此,总体分析表明 130316561 和 134978234 均可用作 TcHGPRT 抑制剂。此外,































 京公网安备 11010802027423号
京公网安备 11010802027423号