Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical Reduction of CO2 Toward C2 Valuables on Cu@Ag Core-Shell Tandem Catalyst with Tunable Shell Thickness
Small ( IF 13.0 ) Pub Date : 2021-08-03 , DOI: 10.1002/smll.202102293 Shuaishuai Zhang 1, 2 , Shulin Zhao 1 , Dongxue Qu 1 , Xiaojing Liu 1 , Yuping Wu 1, 2 , Yuhui Chen 1 , Wei Huang 2
Small ( IF 13.0 ) Pub Date : 2021-08-03 , DOI: 10.1002/smll.202102293 Shuaishuai Zhang 1, 2 , Shulin Zhao 1 , Dongxue Qu 1 , Xiaojing Liu 1 , Yuping Wu 1, 2 , Yuhui Chen 1 , Wei Huang 2
Affiliation
|
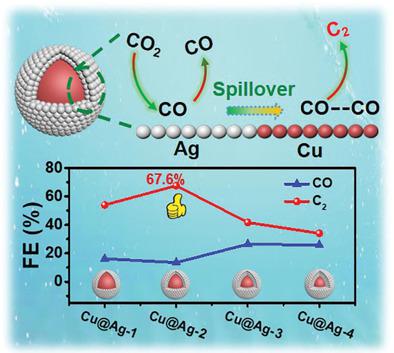
Electrochemical CO2 reduction reaction (CO2RR) is critical to converting CO2 to high-value multicarbon chemicals. However, the Cu-based catalysts as the only option to reduce CO2 into C2+ products suffer from poor selectivity and low activity. Tandem catalysis for CO2 reduction is an efficient strategy to overcome such problems. Here, Cu@Ag core-shell nanoparticles (NPs) with different silver layer thicknesses are fabricated to realize the tandem catalysis for CO2 conversion by producing CO on Ag shell and further achieving C–C coupling on Cu core. It is found that Cu@Ag-2 NPs with the proper thickness of Ag shell exhibit the Faradaic efficiency (FE) of total C2 products and ethylene as high as 67.6% and 32.2% at −1.1 V (versus reversible hydrogen electrode, RHE), respectively. Moreover, it exhibits remarkably electrocatalytic stability after 14 h. Based on electrochemical tests and CO adsorption capacity analyses, the origin of the enhanced catalytic performance can be attributed to the synergistic effect between Ag shell and Cu core, which strengthens the bonding strength of CO on Cu/Ag interfaces, expedites the charge transfer, increases the electrochemical surface areas (ECSAs). This report provides a Cu-based catalyst to realize efficient C2 generation via a rationally designed core-shell structured catalyst.
中文翻译:

壳厚可调的Cu@Ag核壳串联催化剂上CO2向C2贵重物的电化学还原
电化学 CO 2还原反应 (CO 2 RR) 是将 CO 2转化为高价值多碳化学品的关键。然而,Cu基催化剂作为将CO 2还原为C 2+产物的唯一选择存在选择性差和活性低的问题。用于 CO 2还原的串联催化是克服这些问题的有效策略。在这里,制备具有不同银层厚度的 Cu@Ag 核壳纳米粒子 (NPs) 以实现对 CO 2的串联催化通过在银壳上产生 CO 并进一步在铜核上实现 C-C 耦合。发现具有适当厚度的 Ag 壳层的 Cu@Ag-2 NPs在 -1.1 V 下表现出总 C 2产物和乙烯的法拉第效率(FE)高达 67.6% 和 32.2%(相对于可逆氢电极,RHE ), 分别。此外,它在 14 小时后表现出显着的电催化稳定性。基于电化学测试和 CO 吸附容量分析,催化性能增强的原因可以归因于 Ag 壳和 Cu 核之间的协同作用,这增强了 CO 在 Cu/Ag 界面上的结合强度,加快了电荷转移,增加了电化学表面积 (ECSA)。该报告提供了一种基于铜的催化剂,以实现高效的 C 2 通过合理设计的核壳结构催化剂产生。
更新日期:2021-09-16
中文翻译:

壳厚可调的Cu@Ag核壳串联催化剂上CO2向C2贵重物的电化学还原
电化学 CO 2还原反应 (CO 2 RR) 是将 CO 2转化为高价值多碳化学品的关键。然而,Cu基催化剂作为将CO 2还原为C 2+产物的唯一选择存在选择性差和活性低的问题。用于 CO 2还原的串联催化是克服这些问题的有效策略。在这里,制备具有不同银层厚度的 Cu@Ag 核壳纳米粒子 (NPs) 以实现对 CO 2的串联催化通过在银壳上产生 CO 并进一步在铜核上实现 C-C 耦合。发现具有适当厚度的 Ag 壳层的 Cu@Ag-2 NPs在 -1.1 V 下表现出总 C 2产物和乙烯的法拉第效率(FE)高达 67.6% 和 32.2%(相对于可逆氢电极,RHE ), 分别。此外,它在 14 小时后表现出显着的电催化稳定性。基于电化学测试和 CO 吸附容量分析,催化性能增强的原因可以归因于 Ag 壳和 Cu 核之间的协同作用,这增强了 CO 在 Cu/Ag 界面上的结合强度,加快了电荷转移,增加了电化学表面积 (ECSA)。该报告提供了一种基于铜的催化剂,以实现高效的 C 2 通过合理设计的核壳结构催化剂产生。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号