当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Properties of Two-Component Mixtures of Isobutylcyclohexane (1) or tert-Butylcyclohexane (1) with n-Dodecane (2) or n-Hexadecane (2): Densities, Surface Tensions, Viscosities, and Speeds of Sound at 0.1 MPa and Various Temperatures
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-08-03 , DOI: 10.1021/acs.jced.1c00242 Dianne J. Luning Prak 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-08-03 , DOI: 10.1021/acs.jced.1c00242 Dianne J. Luning Prak 1
Affiliation
|
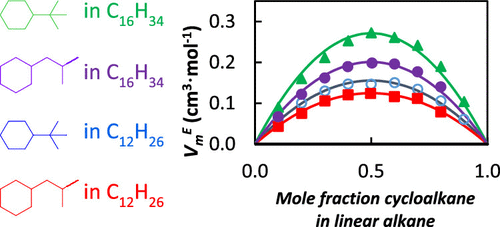
This study provides data on measured densities, viscosities, speeds of sound, surface tensions, and calculated bulk moduli of two-component mixtures of a linear alkane (dodecane or hexadecane) with a branched cyclohexane (isobutyl- and t-butyl-cyclohexane). For most systems tested, the mixture properties changed monotonically from the pure alkane value to the cyclohexane isomer value as the mole fraction of the cyclohexane isomer increased. For some systems, a mixture had the lowest value for a particular property. This was found for the speed of sound of isobutylcyclohexane in n-dodecane, the kinematic viscosities of t-butylcyclohexane in n-dodecane, and the isentropic bulk modulus t-butylcyclohexane in n-hexadecane. Viscosity data were fitted using the correlations from McAllister, Jouyban–Acree, and Grunberg–Nissan. All three correlations fit the data within the experimental uncertainty of the measurements, and neither was clearly the best predictor for all mixture systems. The excess speeds of sound and molar Gibbs energies of activation for viscous flow were smaller than the propagated uncertainty of the calculated value. All viscosity deviations were negative, and the greatest deviation was −0.12 mPa s at 293.15 K. The excess molar volumes (VmE) were positive for all mixtures, and greater values were found in mixtures with the longer alkane and with t-butylcyclohexane. This suggests that the substituent on the cyclohexane that was bulkier did not pack as well as the less bulky “iso” group. The VmE values decreased as temperature increased for all mixtures. These mixture properties can be used by fuel chemists and engineers who are trying to create model mixture systems for fuels that contain linear, branched, cyclic, and aromatic compounds.
中文翻译:

异丁基环己烷 (1) 或叔丁基环己烷 (1) 与正十二烷 (2) 或正十六烷 (2) 的双组分混合物的特性:密度、表面张力、粘度和 0.1 MPa 和各种温度下的声速
本研究提供了线性烷烃(十二烷或十六烷)与支化环己烷(异丁基和叔丁基环己烷)的双组分混合物的测量密度、粘度、声速、表面张力和计算体积模量的数据。对于大多数测试的系统,随着环己烷异构体摩尔分数的增加,混合物性质从纯烷烃值单调变化到环己烷异构体值。对于某些系统,混合物具有特定属性的最低值。此被发现的isobutylcyclohexane的声音在速度Ñ -dodecane,运动的粘度吨-butylcyclohexane在Ñ -dodecane,和等熵体积模量吨-butylcyclohexane在n -十六烷。使用 McAllister、Jouyban-Acree 和 Grunberg-Nissan 的相关性拟合粘度数据。所有三个相关性都在测量的实验不确定性范围内拟合数据,并且显然都不是所有混合系统的最佳预测值。粘性流的超速声速和摩尔吉布斯激活能小于计算值的传播不确定性。所有粘度偏差均为负值,最大偏差为 293.15 K 时的 -0.12 mPa s 。所有混合物的过量摩尔体积 ( V m E ) 均为正值,在具有较长烷烃和t 的混合物中发现更大的值-丁基环己烷。这表明体积较大的环己烷上的取代基不像体积较小的“异”基团那样堆积。对于所有混合物,V m E值随着温度升高而降低。这些混合物特性可供燃料化学家和工程师使用,他们试图为包含线性、支化、环状和芳香族化合物的燃料创建模型混合系统。
更新日期:2021-08-12
中文翻译:

异丁基环己烷 (1) 或叔丁基环己烷 (1) 与正十二烷 (2) 或正十六烷 (2) 的双组分混合物的特性:密度、表面张力、粘度和 0.1 MPa 和各种温度下的声速
本研究提供了线性烷烃(十二烷或十六烷)与支化环己烷(异丁基和叔丁基环己烷)的双组分混合物的测量密度、粘度、声速、表面张力和计算体积模量的数据。对于大多数测试的系统,随着环己烷异构体摩尔分数的增加,混合物性质从纯烷烃值单调变化到环己烷异构体值。对于某些系统,混合物具有特定属性的最低值。此被发现的isobutylcyclohexane的声音在速度Ñ -dodecane,运动的粘度吨-butylcyclohexane在Ñ -dodecane,和等熵体积模量吨-butylcyclohexane在n -十六烷。使用 McAllister、Jouyban-Acree 和 Grunberg-Nissan 的相关性拟合粘度数据。所有三个相关性都在测量的实验不确定性范围内拟合数据,并且显然都不是所有混合系统的最佳预测值。粘性流的超速声速和摩尔吉布斯激活能小于计算值的传播不确定性。所有粘度偏差均为负值,最大偏差为 293.15 K 时的 -0.12 mPa s 。所有混合物的过量摩尔体积 ( V m E ) 均为正值,在具有较长烷烃和t 的混合物中发现更大的值-丁基环己烷。这表明体积较大的环己烷上的取代基不像体积较小的“异”基团那样堆积。对于所有混合物,V m E值随着温度升高而降低。这些混合物特性可供燃料化学家和工程师使用,他们试图为包含线性、支化、环状和芳香族化合物的燃料创建模型混合系统。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号