Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2021-08-02 , DOI: 10.1016/j.jinorgbio.2021.111560 Marlon P Almeida 1 , Flávio V C Kock 1 , Hugo C R de Jesus 2 , Rose M Carlos 1 , Tiago Venâncio 1
|
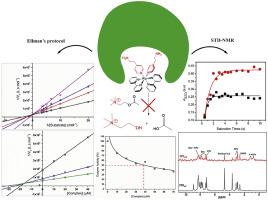
Currently, acetylcholinesterase (AChE) inhibitors are the only anti-Alzheimer drugs commercially available. Despite their wide use those drugs are all dose dependent and their effect last for no longer than two years, with several side effects. The search of novel acetylcholinesterase (AChE) inhibitors remains as the main scientific route. Here we describe the synthesis, characterization, biological activity and an NMR binding-target study of a novel cis-[Ru(Bpy)2(EtPy)2]2+, (RuEtPy), Bpy = 2,2′-bipyridine and EtPy = 4,2-Ethylamino-pyridine) as a potential AChE inhibitor. The classic Ellman's colorimetric assay suggests that the RuEtPy exhibits a high inhibitory activity, following a competitive mechanism, with a remarkable low inhibition constant (Ki ≈ 16.8 μM), together with a IC50 = 39 μM. Hence, we have studied the spatial interactions for this novel candidate towards the human acetylcholinesterase (hAChE) using saturation transfer difference (STD)-NMR, in order to describe the mechanism of the interaction. NMR binding-target results shows that the 4,2-Ethylamino-Pyridine group is spatially closer to hAChE surface chemical arrangement than 2,2′ bipyridine counterpart, exerting an efficient intermolecular interaction, with a low dissociation constant (KD ≈ 55 μM), probing that 4,2-Ethylamino-pyridine motif plays a key role in the inhibitory action.
中文翻译:

(STD)-NMR研究新型Ru(II)多吡啶基配合物的乙酰胆碱酯酶抑制活性及超分子相互作用
目前,乙酰胆碱酯酶 (AChE) 抑制剂是唯一市售的抗阿尔茨海默病药物。尽管它们被广泛使用,但这些药物都是剂量依赖性的,并且它们的效果持续不超过两年,并有一些副作用。寻找新型乙酰胆碱酯酶 (AChE) 抑制剂仍然是主要的科学途径。在这里,我们描述了一种新型cis -[Ru(Bpy) 2 (EtPy) 2 ] 2+的合成、表征、生物活性和 NMR 结合靶点研究, (RuEtPy), Bpy = 2,2'-联吡啶和 EtPy = 4,2-乙基氨基-吡啶) 作为潜在的 AChE 抑制剂。经典的 Ellman 比色分析表明,RuEtPy 表现出高抑制活性,遵循竞争机制,具有显着的低抑制常数 (Ki ≈ 16.8 μM),IC 50 = 39 μM。因此,我们使用饱和转移差 (STD)-NMR 研究了这种新型候选物与人乙酰胆碱酯酶 (hAChE) 的空间相互作用,以描述相互作用的机制。核磁共振结合目标结果表明,4,2-乙氨基吡啶基团比 2,2' 联吡啶对应物在空间上更接近 hAChE 表面化学排列,发挥有效的分子间相互作用,具有低解离常数 (K D ≈ 55 μM),探测 4,2-乙基氨基-吡啶基序在抑制作用中起关键作用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号