当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cancer Cytomembrane-Cloaked Prussian Blue Nanoparticles Enhance the Efficacy of Mild-Temperature Photothermal Therapy by Disrupting Mitochondrial Functions of Cancer Cells
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-08-02 , DOI: 10.1021/acsami.1c11138 Pei Wang 1, 2 , Ranjith Kumar Kankala 1 , Biaoqi Chen 1 , Yang Zhang 3 , Mingzhi Zhu 1 , Xuemei Li 1 , Ruimin Long 1 , Dayun Yang 4 , Rumen Krastev 5 , Shibin Wang 1 , Xin Xiong 6 , Yuangang Liu 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-08-02 , DOI: 10.1021/acsami.1c11138 Pei Wang 1, 2 , Ranjith Kumar Kankala 1 , Biaoqi Chen 1 , Yang Zhang 3 , Mingzhi Zhu 1 , Xuemei Li 1 , Ruimin Long 1 , Dayun Yang 4 , Rumen Krastev 5 , Shibin Wang 1 , Xin Xiong 6 , Yuangang Liu 1
Affiliation
|
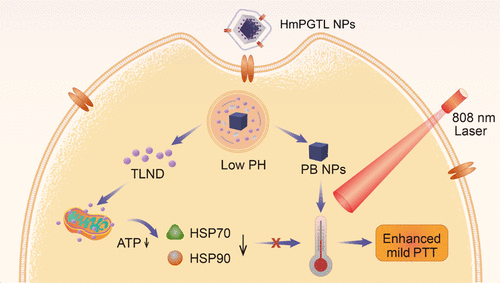
Despite its success against cancer, photothermal therapy (PTT) (>50 °C) suffers from several limitations such as triggering inflammation and facilitating immune escape and metastasis and also damage to the surrounding normal cells. Mild-temperature PTT has been proposed to override these shortcomings. We developed a nanosystem using HepG2 cancer cell membrane-cloaked zinc glutamate-modified Prussian blue nanoparticles with triphenylphosphine-conjugated lonidamine (HmPGTL NPs). This innovative approach achieved an efficient mild-temperature PTT effect by downregulating the production of intracellular ATP. This disrupts a section of heat shock proteins that cushion cancer cells against heat. The physicochemical properties, anti-tumor efficacy, and mechanisms of HmPGTL NPs both in vitro and in vivo were investigated. Moreover, the nanoparticles cloaked with the HepG2 cell membrane substantially prolonged the circulation time in vivo. Overall, the designed nanocomposites enhance the efficacy of mild-temperature PTT by disrupting the production of ATP in cancer cells. Thus, we anticipate that the mild-temperature PTT nanosystem will certainly present its enormous potential in various biomedical applications.
中文翻译:

癌症细胞膜包裹的普鲁士蓝纳米颗粒通过破坏癌细胞的线粒体功能来增强温和光热疗法的功效
尽管光热疗法(PTT)(> 50°C)在抗癌方面取得了成功,但仍存在一些局限性,例如引发炎症、促进免疫逃逸和转移以及对周围正常细胞的损害。温和温度 PTT 已被提出来克服这些缺点。我们开发了一种纳米系统,使用 HepG2 癌细胞膜包裹的谷氨酸锌修饰的普鲁士蓝纳米颗粒和三苯基膦缀合的洛尼达明 (HmPGTL NP)。这种创新方法通过下调细胞内 ATP 的产生,实现了高效的温和温度 PTT 效果。这破坏了热休克蛋白的一部分,该蛋白可以缓冲癌细胞免受热的影响。研究了 HmPGTL NPs 的体外和体内理化性质、抗肿瘤功效和机制。此外,包裹有HepG2细胞膜的纳米粒子大大延长了体内的循环时间。总体而言,设计的纳米复合材料通过破坏癌细胞中 ATP 的产生来增强温和温度 PTT 的功效。因此,我们预计温和的PTT纳米系统必将在各种生物医学应用中展现其巨大的潜力。
更新日期:2021-08-11
中文翻译:

癌症细胞膜包裹的普鲁士蓝纳米颗粒通过破坏癌细胞的线粒体功能来增强温和光热疗法的功效
尽管光热疗法(PTT)(> 50°C)在抗癌方面取得了成功,但仍存在一些局限性,例如引发炎症、促进免疫逃逸和转移以及对周围正常细胞的损害。温和温度 PTT 已被提出来克服这些缺点。我们开发了一种纳米系统,使用 HepG2 癌细胞膜包裹的谷氨酸锌修饰的普鲁士蓝纳米颗粒和三苯基膦缀合的洛尼达明 (HmPGTL NP)。这种创新方法通过下调细胞内 ATP 的产生,实现了高效的温和温度 PTT 效果。这破坏了热休克蛋白的一部分,该蛋白可以缓冲癌细胞免受热的影响。研究了 HmPGTL NPs 的体外和体内理化性质、抗肿瘤功效和机制。此外,包裹有HepG2细胞膜的纳米粒子大大延长了体内的循环时间。总体而言,设计的纳米复合材料通过破坏癌细胞中 ATP 的产生来增强温和温度 PTT 的功效。因此,我们预计温和的PTT纳米系统必将在各种生物医学应用中展现其巨大的潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号