当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lithium-Promoted Cycloaddition of Indole-2,3-dienolates and Carbon Disulfide as a One-Pot Route to Thiopyrano[4,3-b]indole-3(5H)-thiones
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-08-02 , DOI: 10.1021/acs.joc.1c01200 Konstantin F Suzdalev 1 , Julia V Vyalyh 1 , Valery V Tkachev 2 , Ekaterina A Lysenko 1 , Oleg N Burov 1 , Anton V Lisovin 1 , Mikhail E Kletskii 1 , Sergey V Kurbatov 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-08-02 , DOI: 10.1021/acs.joc.1c01200 Konstantin F Suzdalev 1 , Julia V Vyalyh 1 , Valery V Tkachev 2 , Ekaterina A Lysenko 1 , Oleg N Burov 1 , Anton V Lisovin 1 , Mikhail E Kletskii 1 , Sergey V Kurbatov 1
Affiliation
|
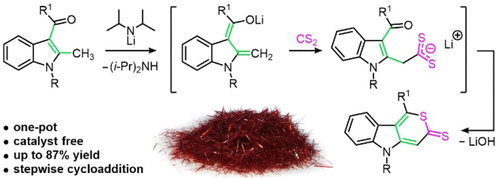
A new approach for the annulation of a thiopyrane ring to an indole core under mild conditions was developed. Treating 2-methyl-3-acylindoles with lithium diisopropyl amide leads to the elimination of a proton from the 2-methyl group. The lithium indole-2,3-dienolates obtained were found to react with CS2 to give the corresponding thiopyrano[4,3-b]indole-3(5H)-thiones. The mechanism represents a stepwise addition through ion-pair formation, according to PCM/B3LYP/6-311++G**, PBE1PBE/6-311++G**, and MP2//HF/6-311++G** quantum chemical calculations. AIM calculations revealed the essential role of the Li atom at all stages of the process.
中文翻译:

锂促进 Indole-2,3-dienolates 和二硫化碳的环加成作为一锅法合成噻吩并 [4,3-b]indole-3(5H)-thiones
开发了一种在温和条件下将噻喃环与吲哚核成环的新方法。用二异丙基氨基锂处理 2-甲基-3-酰基吲哚导致从 2-甲基基团消除一个质子。发现获得的吲哚-2,3-二烯醇锂与CS 2反应得到相应的噻喃并[4,3 - b ]吲哚-3(5 H )-硫酮。根据 PCM/B3LYP/6-311++G**、PBE1PBE/6-311++G** 和 MP2//HF/6-311++G,该机制代表通过离子对形成的逐步加成** 量子化学计算。AIM 计算揭示了锂原子在该过程的所有阶段的重要作用。
更新日期:2021-09-03
中文翻译:

锂促进 Indole-2,3-dienolates 和二硫化碳的环加成作为一锅法合成噻吩并 [4,3-b]indole-3(5H)-thiones
开发了一种在温和条件下将噻喃环与吲哚核成环的新方法。用二异丙基氨基锂处理 2-甲基-3-酰基吲哚导致从 2-甲基基团消除一个质子。发现获得的吲哚-2,3-二烯醇锂与CS 2反应得到相应的噻喃并[4,3 - b ]吲哚-3(5 H )-硫酮。根据 PCM/B3LYP/6-311++G**、PBE1PBE/6-311++G** 和 MP2//HF/6-311++G,该机制代表通过离子对形成的逐步加成** 量子化学计算。AIM 计算揭示了锂原子在该过程的所有阶段的重要作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号