当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Influence of stereochemistry on the activity of rapadocin, an isoform-specific inhibitor of the nucleoside transporter ENT1
Chemical Science ( IF 7.6 ) Pub Date : 2021-07-21 , DOI: 10.1039/d1sc02295d Yuefan Wang 1, 2 , Hanjing Peng 1, 2 , Zufeng Guo 1, 2, 3 , Brett R Ullman 4 , Kana Yamamoto 4 , Sam Y Hong 4 , Jun O Liu 1, 2, 5
Chemical Science ( IF 7.6 ) Pub Date : 2021-07-21 , DOI: 10.1039/d1sc02295d Yuefan Wang 1, 2 , Hanjing Peng 1, 2 , Zufeng Guo 1, 2, 3 , Brett R Ullman 4 , Kana Yamamoto 4 , Sam Y Hong 4 , Jun O Liu 1, 2, 5
Affiliation
|
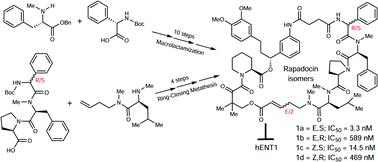
Rapadocin is a novel rapamycin-inspired polyketide–tetrapeptide hybrid macrocycle that possesses highly potent and isoform-specific inhibitory activity against the human equilibrative nucleoside transporter 1 (hENT1). Rapadocin contains an epimerizable chiral center in phenylglycine and an olefin group, and can thus exist as a mixture of four stereoisomers. Herein, we report the first total synthesis of the four stereoisomers of rapadocin using two different synthetic strategies and the assignment of their structures. The inhibitory activity of each of the four synthetic isomers on both hENT1 and hENT2 was determined. It was found that the stereochemistry of phenylglycine played a more dominant role than the configuration of the olefin in the activity of rapadocin. These findings will guide the future design and development of rapadocin analogs as new modulators of adenosine signaling.
中文翻译:

立体化学对 rapadocin 活性的影响,核苷转运蛋白 ENT1 的异构体特异性抑制剂
Rapadocin 是一种新型的受雷帕霉素启发的聚酮化合物-四肽杂化大环化合物,对人类平衡核苷转运蛋白 1 (hENT1) 具有高效和同种型特异性抑制活性。Rapadocin 在苯基甘氨酸中包含一个可差向异构的手性中心和一个烯烃基团,因此可以作为四种立体异构体的混合物存在。在此,我们报告了使用两种不同合成策略的 rapadocin 四种立体异构体的首次全合成及其结构分配。测定了四种合成异构体中的每一种对 hENT1 和 hENT2 的抑制活性。发现苯基甘氨酸的立体化学在rapadocin的活性中比烯烃的构型起主导作用。
更新日期:2021-08-02
中文翻译:

立体化学对 rapadocin 活性的影响,核苷转运蛋白 ENT1 的异构体特异性抑制剂
Rapadocin 是一种新型的受雷帕霉素启发的聚酮化合物-四肽杂化大环化合物,对人类平衡核苷转运蛋白 1 (hENT1) 具有高效和同种型特异性抑制活性。Rapadocin 在苯基甘氨酸中包含一个可差向异构的手性中心和一个烯烃基团,因此可以作为四种立体异构体的混合物存在。在此,我们报告了使用两种不同合成策略的 rapadocin 四种立体异构体的首次全合成及其结构分配。测定了四种合成异构体中的每一种对 hENT1 和 hENT2 的抑制活性。发现苯基甘氨酸的立体化学在rapadocin的活性中比烯烃的构型起主导作用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号