当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Self-Contained Photoacid Generator Triggered by Photocyclization of Triangle Terarylene Backbone
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2015-06-01 , DOI: 10.1021/jacs.5b02826 Takuya Nakashima 1 , Kenta Tsuchie 1 , Rui Kanazawa 1 , Ruiji Li 1 , Shunsuke Iijima 1 , Olivier Galangau 2 , Hisako Nakagawa 3 , Katsuya Mutoh 3 , Yoichi Kobayashi 3 , Jiro Abe 3 , Tsuyoshi Kawai 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2015-06-01 , DOI: 10.1021/jacs.5b02826 Takuya Nakashima 1 , Kenta Tsuchie 1 , Rui Kanazawa 1 , Ruiji Li 1 , Shunsuke Iijima 1 , Olivier Galangau 2 , Hisako Nakagawa 3 , Katsuya Mutoh 3 , Yoichi Kobayashi 3 , Jiro Abe 3 , Tsuyoshi Kawai 1, 2
Affiliation
|
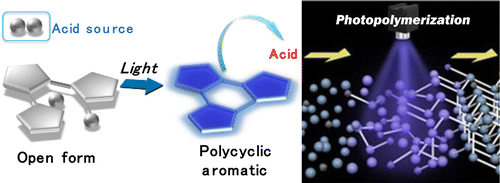
We herein propose a new type of efficient neutral photoacid generator. A photoinduced 6π-electrocyclization reaction of photochromic triangle terarylenes triggers subsequent release of a Brønsted acid, which took place from the photocyclized form. A H-atom and its conjugate base were introduced at both sides of a 6π-system to form the self-contained photoacid generator. UV irradiation to the 6π-system produces a cyclohexa-1,3-diene part with a H-atom and a conjugate base on the sp(3) C-atoms at 5- and 6-positions, respectively, which spontaneously release an acid molecule quantitatively forming a polyaromatic compound. A net quantum yield of photoacid generation as high as 0.52 under ambient conditions and a photoinitiated cationic polymerization of an epoxy monomer are demonstrated.
中文翻译:

三角四芳基主链光环化引发的自含式光酸发生剂
我们在此提出了一种新型高效的中性光酸产生剂。光致变色三角四萘嵌苯的光诱导 6π-电环化反应触发了布朗斯台德酸的后续释放,该反应从光环化形式发生。在 6π 体系的两侧引入 H 原子及其共轭碱以形成自含的光酸产生剂。对 6π 系统的紫外线照射会产生一个带有 H 原子的环己-1,3-二烯部分和分别位于 sp(3) C-原子的 5 位和 6 位的共轭碱,这会自发地释放酸分子定量形成多环芳烃化合物。证明了在环境条件下光酸产生的净量子产率高达 0.52,并证明了环氧单体的光引发阳离子聚合。
更新日期:2015-06-01
中文翻译:

三角四芳基主链光环化引发的自含式光酸发生剂
我们在此提出了一种新型高效的中性光酸产生剂。光致变色三角四萘嵌苯的光诱导 6π-电环化反应触发了布朗斯台德酸的后续释放,该反应从光环化形式发生。在 6π 体系的两侧引入 H 原子及其共轭碱以形成自含的光酸产生剂。对 6π 系统的紫外线照射会产生一个带有 H 原子的环己-1,3-二烯部分和分别位于 sp(3) C-原子的 5 位和 6 位的共轭碱,这会自发地释放酸分子定量形成多环芳烃化合物。证明了在环境条件下光酸产生的净量子产率高达 0.52,并证明了环氧单体的光引发阳离子聚合。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号