当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of Kadcoccinic Acid A Trimethyl Ester
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-07-29 , DOI: 10.1021/jacs.1c05521 Barry M Trost 1 , Guoting Zhang 1 , Hadi Gholami 1 , Daniel Zell 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-07-29 , DOI: 10.1021/jacs.1c05521 Barry M Trost 1 , Guoting Zhang 1 , Hadi Gholami 1 , Daniel Zell 1
Affiliation
|
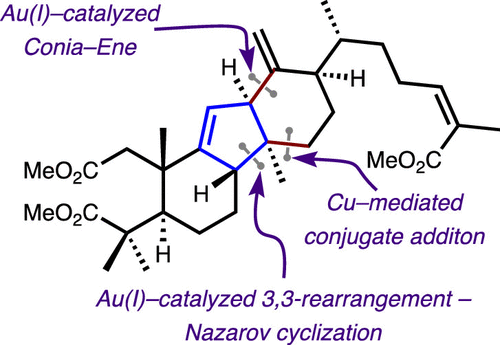
The first total synthesis of the trimethyl ester of kadcoccinic acid A is described. The central structural element of our synthesis is a cyclopentenone motif that allows the assembly of the natural product skeleton. A gold(I)-catalyzed cyclization of an enynyl acetate led to efficient construction of the cyclopentenone scaffold. In this step, optimization studies revealed that the stereochemistry of the enynyl acetate dictates regioisomeric cyclopentenone formation. The synthesis further highlights an efficient copper-mediated conjugate addition, merged with a gold(I)-catalyzed Conia-ene reaction to connect the two fragments, thereby forging the D-ring of the natural product. The synthetic strategy reported herein can provide a general platform to access the skeleton of other members of this family of natural products.
中文翻译:

Kadcoccinic Acid A三甲酯的全合成
描述了kadcoccinic酸A的三甲酯的第一次全合成。我们合成的核心结构元素是允许组装天然产物骨架的环戊烯酮基序。金(I)催化的乙酸烯基环化导致环戊烯酮支架的有效构建。在这一步中,优化研究表明,乙酸烯炔酯的立体化学决定了区域异构环戊烯酮的形成。该合成进一步突出了有效的铜介导的共轭加成,与金 (I) 催化的 Conia-ene 反应合并以连接两个片段,从而形成天然产物的 D 环。本文报道的合成策略可以提供一个通用平台来访问该天然产物家族其他成员的骨架。
更新日期:2021-08-11
中文翻译:

Kadcoccinic Acid A三甲酯的全合成
描述了kadcoccinic酸A的三甲酯的第一次全合成。我们合成的核心结构元素是允许组装天然产物骨架的环戊烯酮基序。金(I)催化的乙酸烯基环化导致环戊烯酮支架的有效构建。在这一步中,优化研究表明,乙酸烯炔酯的立体化学决定了区域异构环戊烯酮的形成。该合成进一步突出了有效的铜介导的共轭加成,与金 (I) 催化的 Conia-ene 反应合并以连接两个片段,从而形成天然产物的 D 环。本文报道的合成策略可以提供一个通用平台来访问该天然产物家族其他成员的骨架。































 京公网安备 11010802027423号
京公网安备 11010802027423号