当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cage-Expansion of Fullerenes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-07-29 , DOI: 10.1021/jacs.1c05778 Sheng Zhang 1 , Yoshifumi Hashikawa 1 , Yasujiro Murata 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-07-29 , DOI: 10.1021/jacs.1c05778 Sheng Zhang 1 , Yoshifumi Hashikawa 1 , Yasujiro Murata 1
Affiliation
|
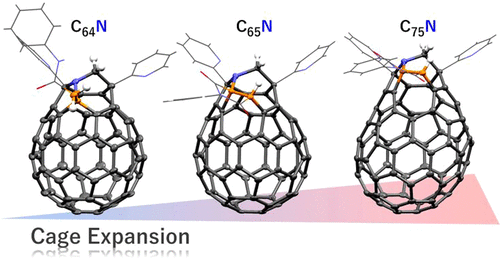
Despite the first proposal on the cage inflation of fullerenes in 1991, the chemical expansion of fullerenes has been still a formidable challenge. Herein, we provide an efficient methodology to expand [60] and [70]fullerene cages by the inclusion of totally C5N unit, giving nitrogen-containing closed structures as C65N and C75N with double fused heptagons. This method consists of two steps commenced with the construction of an opening by the reaction with triazine as a C3N source, followed by the cage reformation using N-phenylmaleimide as a C2 source. We also synthesized endohedral cages, demonstrating that the encapsulated H2O molecule inside the C75N cage prefers the orientation which maximizes the intramolecular interaction with the carbon wall. Additionally, we revealed the existence of a through-space magnetic dipolar interaction between the encapsulated H2 molecule and the embedded N atom.
中文翻译:

富勒烯的笼式膨胀
尽管 1991 年首次提出了富勒烯笼式膨胀,但富勒烯的化学膨胀仍然是一项艰巨的挑战。在此,我们提供了一种有效的方法,通过包含完全 C5N 单元来扩展 [60] 和 [70] 富勒烯笼,给出含氮封闭结构,如具有双稠合七边形的C 65 N 和 C 75 N。该方法由两个步骤组成,首先通过与作为 C3N 源的三嗪反应构建开口,然后使用N-苯基马来酰亚胺作为 C2 源进行笼形改造。我们还合成了内嵌笼,证明C 75内部封装的 H 2 O 分子N笼更喜欢使与碳壁的分子内相互作用最大化的取向。此外,我们揭示了封装的 H 2分子和嵌入的 N 原子之间存在穿越空间的磁偶极相互作用。
更新日期:2021-08-19
中文翻译:

富勒烯的笼式膨胀
尽管 1991 年首次提出了富勒烯笼式膨胀,但富勒烯的化学膨胀仍然是一项艰巨的挑战。在此,我们提供了一种有效的方法,通过包含完全 C5N 单元来扩展 [60] 和 [70] 富勒烯笼,给出含氮封闭结构,如具有双稠合七边形的C 65 N 和 C 75 N。该方法由两个步骤组成,首先通过与作为 C3N 源的三嗪反应构建开口,然后使用N-苯基马来酰亚胺作为 C2 源进行笼形改造。我们还合成了内嵌笼,证明C 75内部封装的 H 2 O 分子N笼更喜欢使与碳壁的分子内相互作用最大化的取向。此外,我们揭示了封装的 H 2分子和嵌入的 N 原子之间存在穿越空间的磁偶极相互作用。















































 京公网安备 11010802027423号
京公网安备 11010802027423号